Synaptic protection in the brain of WldS mice occurs independently of age but is sensitive to gene-dose
- PMID: 21124744
- PMCID: PMC2993971
- DOI: 10.1371/journal.pone.0015108
Synaptic protection in the brain of WldS mice occurs independently of age but is sensitive to gene-dose
Abstract
Background: Disruption of synaptic connectivity is a significant early event in many neurodegenerative conditions affecting the aging CNS, including Alzheimer's disease and Parkinson's disease. Therapeutic approaches that protect synapses from degeneration in the aging brain offer the potential to slow or halt the progression of such conditions. A range of animal models expressing the slow Wallerian Degeneration (Wld(S)) gene show robust neuroprotection of synapses and axons from a wide variety of traumatic and genetic neurodegenerative stimuli in both the central and peripheral nervous systems, raising that possibility that Wld(S) may be useful as a neuroprotective agent in diseases with synaptic pathology. However, previous studies of neuromuscular junctions revealed significant negative effects of increasing age and positive effects of gene-dose on Wld(S)-mediated synaptic protection in the peripheral nervous system, raising doubts as to whether Wld(S) is capable of directly conferring synapse protection in the aging brain.
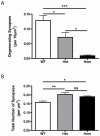
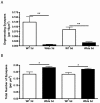
Methodology/principal findings: We examined the influence of age and gene-dose on synaptic protection in the brain of mice expressing the Wld(S) gene using an established cortical lesion model to induce synaptic degeneration in the striatum. Synaptic protection was found to be sensitive to Wld(S) gene-dose, with heterozygous Wld(S) mice showing approximately half the level of protection observed in homozygous Wld(S) mice. Increasing age had no influence on levels of synaptic protection. In contrast to previous findings in the periphery, synapses in the brain of old Wld(S) mice were just as strongly protected as those in young mice.
Conclusions/significance: Our study demonstrates that Wld(S)-mediated synaptic protection in the CNS occurs independently of age, but is sensitive to gene dose. This suggests that the Wld(S) gene, and in particular its downstream endogenous effector pathways, may be potentially useful therapeutic agents for conferring synaptic protection in the aging brain.
Conflict of interest statement
Figures




References
-
- Wishart TM, Parson SH, Gillingwater TH. Synaptic vulnerability in neurodegenerative disease. J Neuropathol Exp Neurol. 2006;65:733–739. - PubMed
-
- Selkoe DJ. Alzheimer's disease is a synaptic failure. Science. 2002;298:789–791. - PubMed
-
- Jeffrey M, Halliday WG, Bell J, Johnston AR, MacLeod NK, et al. Synapse loss associated with abnormal PrP precedes neuronal degeneration in the scrapie-infected murine hippocampus. Neuropathol Appl Neurobiol. 2000;26:41–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

