Estrogen receptor β exerts tumor repressive functions in human malignant pleural mesothelioma via EGFR inactivation and affects response to gefitinib
- PMID: 21124760
- PMCID: PMC2993924
- DOI: 10.1371/journal.pone.0014110
Estrogen receptor β exerts tumor repressive functions in human malignant pleural mesothelioma via EGFR inactivation and affects response to gefitinib
Abstract
Background: The role of estrogen and estrogen receptors in oncogenesis has been investigated in various malignancies. Recently our group identified estrogen receptor beta (ERβ) expression as an independent prognostic factor in the progression of human Malignant Pleural Mesothelioma (MMe), but the underlying mechanism by which ERβ expression in tumors determines clinical outcome remains largely unknown. This study is aimed at investigating the molecular mechanisms of ERβ action in MMe cells and disclosing the potential translational implications of these results.
Methods: We modulated ERβ expression in REN and MSTO-211H MMe cell lines and evaluated cell proliferation and EGF receptor (EGFR) activation.
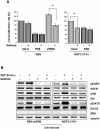
Results: Our data indicate that ERβ knockdown in ER positive cells confers a more invasive phenotype, increases anchorage independent proliferation and elevates the constitutive activation of EGFR-coupled signal transduction pathways. Conversely, re-expression of ERβ in ER negative cells confers a more epithelioid phenotype, decreases their capacity for anchorage independent growth and down-modulates proliferative signal transduction pathways. We identify a physical interaction between ERβ, EGFR and caveolin 1 that results in an altered internalization and in a selective reduced activation of EGFR-coupled signaling, when ERβ is over-expressed. We also demonstrate that differential expression of ERβ influences MMe tumor cell responsiveness to the therapeutic agent: Gefitinib.
Conclusions: This study describes a role for ERβ in the modulation of cell proliferation and EGFR activation and provides a rationale to facilitate the targeting of a subgroup of MMe patients who would benefit most from therapy with Gefitinib alone or in combination with Akt inhibitors.
Conflict of interest statement
Figures




References
-
- Pass HI, Carbone M. Current status of screening for malignant pleural mesothelioma. Semin Thorac Cardiovasc Surg. 2009;21:97–104. - PubMed
-
- Richards WG. Recent advances in mesothelioma staging. Semin Thorac Cardiovasc Surg. 2009;21:105–10. - PubMed
-
- Steele JP. Prognostic factors for mesothelioma. Hematol Oncol Clin North Am. 2005;19:1041–52. Review. - PubMed
-
- Pinton G, Brunelli E, Murer B, Puntoni R, Puntoni M, et al. Oestrogen receptor β impacts on prognosis of human malignant mesothelioma. Cancer Res. 2009;69:4598–604. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

