Glycosylation focuses sequence variation in the influenza A virus H1 hemagglutinin globular domain
- PMID: 21124818
- PMCID: PMC2991263
- DOI: 10.1371/journal.ppat.1001211
Glycosylation focuses sequence variation in the influenza A virus H1 hemagglutinin globular domain
Abstract
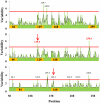
Antigenic drift in the influenza A virus hemagglutinin (HA) is responsible for seasonal reformulation of influenza vaccines. Here, we address an important and largely overlooked issue in antigenic drift: how does the number and location of glycosylation sites affect HA evolution in man? We analyzed the glycosylation status of all full-length H1 subtype HA sequences available in the NCBI influenza database. We devised the "flow index" (FI), a simple algorithm that calculates the tendency for viruses to gain or lose consensus glycosylation sites. The FI predicts the predominance of glycosylation states among existing strains. Our analyses show that while the number of glycosylation sites in the HA globular domain does not influence the overall magnitude of variation in defined antigenic regions, variation focuses on those regions unshielded by glycosylation. This supports the conclusion that glycosylation generally shields HA from antibody-mediated neutralization, and implies that fitness costs in accommodating oligosaccharides limit virus escape via HA hyperglycosylation.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Skehel JJ, Wiley DC. Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annu Rev Biochem. 2000;69:531–569. - PubMed
-
- Yewdell JW, Yellen A, Bachi T. Monoclonal antibodies localize events in the folding, assembly, and intracellular transport of the influenza virus hemagglutinin glycoprotein. Cell. 1988;52:843–852. - PubMed
-
- Daniels R, Kurowski B, Johnson AE, Hebert DN. N-linked glycans direct the cotranslational folding pathway of influenza hemagglutinin. Mol Cell. 2003;11:79–90. - PubMed
-
- Wilson IA, Skehel JJ, Wiley DC. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 A resolution. Nature. 1981;289:366–373. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

