Evolution of allosteric citrate binding sites on 6-phosphofructo-1-kinase
- PMID: 21124851
- PMCID: PMC2990764
- DOI: 10.1371/journal.pone.0015447
Evolution of allosteric citrate binding sites on 6-phosphofructo-1-kinase
Abstract
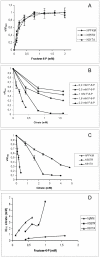
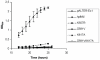
As an important part of metabolism, metabolic flux through the glycolytic pathway is tightly regulated. The most complex control is exerted on 6-phosphofructo-1-kinase (PFK1) level; this control overrules the regulatory role of other allosteric enzymes. Among other effectors, citrate has been reported to play a vital role in the suppression of this enzyme's activity. In eukaryotes, amino acid residues forming the allosteric binding site for citrate are found both on the N- and the C-terminal region of the enzyme. These site has evolved from the phosphoenolpyruvate/ADP binding site of bacterial PFK1 due to the processes of duplication and tandem fusion of prokaryotic ancestor gene followed by the divergence of the catalytic and effector binding sites. Stricter inhibition of the PFK1 enzyme was needed during the evolution of multi-cellular organisms, and the most stringent control of PFK1 by citrate occurs in vertebrates. By substituting a single amino acid (K557R or K617A) as a component of the allosteric binding site in the C-terminal region of human muscle type PFK-M with a residue found in the corresponding site of a fungal enzyme, the inhibitory effect of citrate was attenuated. Moreover, the proteins carrying these single mutations enabled growth of E. coli transformants encoding mutated human PFK-M in a glucose-containing medium that did not support the growth of E. coli transformed with native human PFK-M. Substitution of another residue at the citrate-binding site (D591V) of human PFK-M resulted in the complete loss of activity. Detailed analyses revealed that the mutated PFK-M subunits formed dimers but were unable to associate into the active tetrameric holoenzyme. These results suggest that stricter control over glycolytic flux developed in metazoans, whose somatic cells are largely characterized by slow proliferation.
Conflict of interest statement
Figures

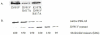
References
-
- Dunaway GA. A review of animal phosphofructokinase isozymes with an emphasis on their physiological role. Mol Cell Biochem. 1983;52(1):75–91. - PubMed
-
- Mertens E. Pyrophosphate-dependent phosphofructokinase, an anaerobic glycolytic enzyme? FEBS Lett. 1991;285(1):1–5. - PubMed
-
- Poorman RA, Randolph A, Kemp RG, Heinrikson RL. Evolution of phosphofructokinase-gene duplication and creation of new effector sites. Nature. 1984;309:467–469. - PubMed
-
- Kemp RG, Foe LG. Allosteric regulatory properties of muscle phosphofructokinase. Mol Cell Biochem. 1983;57:147–154. - PubMed
-
- Li Y, Rivera D, Ru W, Gunasekera D, Kemp RG. Identification of allosteric sites in rabbit phosphofructo-1-kinase. Biochemistry. 1999;38(49):16407–16412. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

