Stimulus-specific adaptation in the auditory thalamus of the anesthetized rat
- PMID: 21124913
- PMCID: PMC2988819
- DOI: 10.1371/journal.pone.0014071
Stimulus-specific adaptation in the auditory thalamus of the anesthetized rat
Abstract
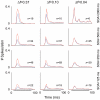
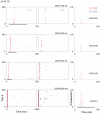
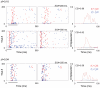
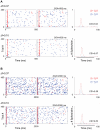
The specific adaptation of neuronal responses to a repeated stimulus (Stimulus-specific adaptation, SSA), which does not fully generalize to other stimuli, provides a mechanism for emphasizing rare and potentially interesting sensory events. Previous studies have demonstrated that neurons in the auditory cortex and inferior colliculus show SSA. However, the contribution of the medial geniculate body (MGB) and its main subdivisions to SSA and detection of rare sounds remains poorly characterized. We recorded from single neurons in the MGB of anaesthetized rats while presenting a sequence composed of a rare tone presented in the context of a common tone (oddball sequences). We demonstrate that a significant percentage of neurons in MGB adapt in a stimulus-specific manner. Neurons in the medial and dorsal subdivisions showed the strongest SSA, linking this property to the non-lemniscal pathway. Some neurons in the non-lemniscal regions showed strong SSA even under extreme testing conditions (e.g., a frequency interval of 0.14 octaves combined with a stimulus onset asynchrony of 2000 ms). Some of these neurons were able to discriminate between two very close frequencies (frequency interval of 0.057 octaves), revealing evidence of hyperacuity in neurons at a subcortical level. Thus, SSA is expressed strongly in the rat auditory thalamus and contribute significantly to auditory change detection.
Conflict of interest statement
Figures









References
-
- Baeuerle P, Von Der Behrens W, Gaese B, Kossl M. Stimulus-Specific Adaptation in the Auditory Thalamus of the Mongolian Gerbil. ARO abstr. 2009;33:646.
-
- Kraus N, McGee T, Littman T, Nicol T, King C. Nonprimary auditory thalamic representation of acoustic change. J Neurophysiol. 1994;72:1270–1277. - PubMed
-
- Nelken I, Ulanovsky N. Mismatch negativity and stimulus-specific adaptation in animal models. Journal of Psycophysiology. 2007;214-223
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

