Fcγ receptor I alpha chain (CD64) expression in macrophages is critical for the onset of meningitis by Escherichia coli K1
- PMID: 21124939
- PMCID: PMC2987830
- DOI: 10.1371/journal.ppat.1001203
Fcγ receptor I alpha chain (CD64) expression in macrophages is critical for the onset of meningitis by Escherichia coli K1
Abstract
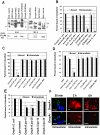
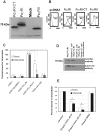
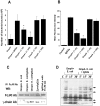
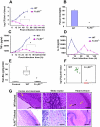
Neonatal meningitis due to Escherichia coli K1 is a serious illness with unchanged morbidity and mortality rates for the last few decades. The lack of a comprehensive understanding of the mechanisms involved in the development of meningitis contributes to this poor outcome. Here, we demonstrate that depletion of macrophages in newborn mice renders the animals resistant to E. coli K1 induced meningitis. The entry of E. coli K1 into macrophages requires the interaction of outer membrane protein A (OmpA) of E. coli K1 with the alpha chain of Fcγ receptor I (FcγRIa, CD64) for which IgG opsonization is not necessary. Overexpression of full-length but not C-terminal truncated FcγRIa in COS-1 cells permits E. coli K1 to enter the cells. Moreover, OmpA binding to FcγRIa prevents the recruitment of the γ-chain and induces a different pattern of tyrosine phosphorylation of macrophage proteins compared to IgG2a induced phosphorylation. Of note, FcγRIa(-/-) mice are resistant to E. coli infection due to accelerated clearance of bacteria from circulation, which in turn was the result of increased expression of CR3 on macrophages. Reintroduction of human FcγRIa in mouse FcγRIa(-/-) macrophages in vitro increased bacterial survival by suppressing the expression of CR3. Adoptive transfer of wild type macrophages into FcγRIa(-/-) mice restored susceptibility to E. coli infection. Together, these results show that the interaction of FcγRI alpha chain with OmpA plays a key role in the development of neonatal meningitis by E. coli K1.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Hornef MW, Wick MJ, Rhen M, Normark S. Bacterial strategies for overcoming host innate and adaptive immune responses. Nat Immunol. 2002;3:1033–1040. - PubMed
-
- Sansonetti PJ. Phagocytosis, a cell biology view. J Cell Sci. 2000;113:3355–3356.
-
- Underhill DM, Ozinsky A. Phagocytosis of microbes: complexity in action. Annu Rev Immunol. 2002;20:825–852. - PubMed
-
- Koedel U, Pfister HW. Models of experimental bacterial meningitis. Role and limitations. Infect Dis Clin North Am. 1999;13:549–577. - PubMed
-
- Leib SL, Tauber MG. Pathogenesis of bacterial meningitis. Infect Dis Clin North Am. 1999;13:527–548. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

