The genetic basis of non-syndromic intellectual disability: a review
- PMID: 21124998
- PMCID: PMC2974911
- DOI: 10.1007/s11689-010-9055-2
The genetic basis of non-syndromic intellectual disability: a review
Abstract
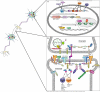
Intellectual disability (ID), also referred to as mental retardation (MR), is frequently the result of genetic mutation. Where ID is present together with additional clinical symptoms or physical anomalies, there is often sufficient information available for the diagnosing physician to identify a known syndrome, which may then educe the identification of the causative defect. However, where co-morbid features are absent, narrowing down a specific gene can only be done by 'brute force' using the latest molecular genetic techniques. Here we attempt to provide a systematic review of genetic causes of cases of ID where no other symptoms or co-morbid features are present, or non-syndromic ID. We attempt to summarize commonalities between the genes and the molecular pathways of their encoded proteins. Since ID is a common feature of autism, and conversely autistic features are frequently present in individuals with ID, we also look at possible overlaps in genetic etiology with non-syndromic ID.
Figures
References
-
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders, Text Revision. 4. Washington: American Psychiatric Association; 2000.
LinkOut - more resources
Full Text Sources
Other Literature Sources
