To eat or not to eat: neuronal metabolism, mitophagy, and Parkinson's disease
- PMID: 21126205
- PMCID: PMC3078495
- DOI: 10.1089/ars.2010.3763
To eat or not to eat: neuronal metabolism, mitophagy, and Parkinson's disease
Abstract
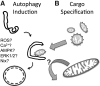
Neurons are exquisitely dependent upon mitochondrial respiration to support energy-demanding functions. Mechanisms that regulate mitochondrial quality control have recently taken center stage in Parkinson's disease research, particularly the selective degradation of mitochondria by autophagy (mitophagy). Unlike other cells, neurons show limited glycolytic potential, and both insufficient and excessive mitophagy have been linked to neurodegeneration. Kinases implicated in regulating mammalian mitophagy include extracellular signal-regulated protein kinases (ERK1/2) and PTEN-induced kinase 1 (PINK1). Increased expression of full-length PINK1 enhances recruitment of parkin to chemically depolarized mitochondria, resulting in rapid mitochondrial clearance in transformed cell lines. As parkin and PINK1 mutations cause autosomal recessive parkinsonism, potential defects in clearing dysfunctional mitochondria may contribute to mitochondrial abnormalities in disease. Given the unique features of metabolic regulation in neurons, however, mechanisms regulating mitochondrial network stability and the threshold for mitophagy are likely to vary from cells that preferentially utilize aerobic glycolysis. Moreover, removal of the entire mitochondrial complement may represent part of a neuronal cell death pathway. Future work utilizing physiological injuries that affect only a subset of mitochondria would help to elucidate whether defective recognition of damaged mitochondria, or alternatively, inability to maintain or generate healthy mitochondria, play the major roles in parkinsonian neurodegeneration.
Figures

References
-
- Bayir H. Kapralov AA. Jiang J. Huang Z. Tyurina YY. Tyurin VA. Zhao Q. Belikova NA. Vlasova II. Maeda A. Zhu J. Na HM. Mastroberardino PG. Sparvero LJ. Amoscato AA. Chu CT. Greenamyre JT. Kagan VE. Peroxidase mechanism of lipid-dependent cross-linking of synuclein with cytochrome C: Protection against apoptosis versus delayed oxidative stress in Parkinson disease. J Biol Chem. 2009;284:15951–15969. - PMC - PubMed
-
- Braak H. Del Tredici K. Poor and protracted myelination as a contributory factor to neurodegenerative disorders. Neurobiol Aging. 2004;25:19–23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

