Targeting the oncogenic protein beta-catenin to enhance chemotherapy outcome against solid human cancers
- PMID: 21126356
- PMCID: PMC3014904
- DOI: 10.1186/1476-4598-9-310
Targeting the oncogenic protein beta-catenin to enhance chemotherapy outcome against solid human cancers
Abstract
Background: Beta-catenin is a multifunctional oncogenic protein that contributes fundamentally to cell development and biology. Elevation in expression and activity of β-catenin has been implicated in many cancers and associated with poor prognosis. Beta-catenin is degraded in the cytoplasm by glycogen synthase kinase 3 beta (GSK-3β) through phosphorylation. Cell growth and proliferation is associated with β-catenin translocation from the cytoplasm into the nucleus. This laboratory was the first to demonstrate that selenium-containing compounds can enhance the efficacy and cytotoxicity of anticancer drugs in several preclinical xenograft models. These data provided the basis to identify mechanism of selenium action focusing on β-catenin as a target. This study was designed to: (1) determine whether pharmacological doses of methylseleninic acid (MSeA) have inhibitory effects on the level and the oncogenic activity of β-catenin, (2) investigate the kinetics and the mechanism of β-catenin inhibition, and (3) confirm that inhibition of β-catenin would lead to enhanced cytotoxicity of standard chemotherapeutic drugs.
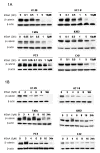
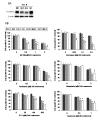
Results: In six human cancer cell lines, the inhibition of total and nuclear expression of β-catenin by MSeA was dose and time dependent. The involvement of GSK-3β in the degradation of β-catenin was cell type dependent (GSK-3β-dependent in HT-29, whereas GSK-3β-independent in HCT-8). However, the pronounced inhibition of β-catenin by MSeA was independent of various drug treatments and was not reversed after combination therapy.Knockout of β-catenin by ShRNA and its inhibition by MSeA yielded similar enhancement of cytotoxicity of anticancer drugs.Collectively, the generated data demonstrate that β-catenin is a target of MSeA and its inhibition resulted in enhanced cytotoxicity of chemotherapeutic drugs.
Conclusions: This study demonstrates that β-catenin, a molecule associated with drug resistance, is a target of selenium and its inhibition is associated with increased multiple drugs cytotoxicity in various human cancers. Further, degradation of β-catenin by GSK-3β is not a general mechanism but is cell type dependent.
Figures




References
-
- Giles RH, van Es JH, Clevers H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta. 2003;1653:1–24. - PubMed
-
- Goto M, Mitra RS, Liu M, Lee J, Henson BS, Carey T, Bradford C, Prince M, Wang CY, Fearon ER, D'Silva NJ. Rap1 stabilizes beta-catenin and enhances beta-catenin-dependent transcription and invasion in squamous cell carcinoma of the head and neck. Clin Cancer Res. 2010;16:65–76. doi: 10.1158/1078-0432.CCR-09-1122. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

