Inhibition and genetic ablation of the B7/CD28 T-cell costimulation axis prevents experimental hypertension
- PMID: 21126972
- PMCID: PMC3064430
- DOI: 10.1161/CIRCULATIONAHA.109.930446
Inhibition and genetic ablation of the B7/CD28 T-cell costimulation axis prevents experimental hypertension
Abstract
Background: The pathogenesis of hypertension remains poorly understood, and treatment is often unsuccessful. Recent evidence suggests that the adaptive immune response plays an important role in this disease. Various hypertensive stimuli cause T-cell activation and infiltration into target organs such as the vessel and the kidney, which promotes vascular dysfunction and blood pressure elevation. Classically, T-cell activation requires T-cell receptor ligation and costimulation. The latter often involves interaction between B7 ligands (CD80 and CD86) on antigen-presenting cells with the T-cell coreceptor CD28. This study was therefore performed to examine the role of this pathway in hypertension.
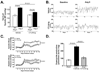
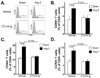
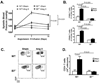
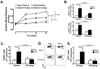
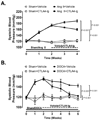
Methods and results: Angiotensin II-induced hypertension increased the presence of activated (CD86(+)) dendritic cells in secondary lymphatic tissues. Blockade of B7-dependent costimulation with CTLA4-Ig reduced both angiotensin II- and deoxycorticosterone acetate (DOCA)-salt-induced hypertension. Activation of circulating T cells, T-cell cytokine production, and vascular T-cell accumulation caused by these hypertensive stimuli were abrogated by CTLA4-Ig. Furthermore, in mice lacking B7 ligands, angiotensin II caused minimal blood pressure elevation and vascular inflammation, and these effects were restored by transplantation with wild-type bone marrow.
Conclusions: T-cell costimulation via B7 ligands is essential for development of experimental hypertension, and inhibition of this process could have therapeutic benefit in the treatment of this disease.
Figures



Comment in
-
T cells and blood vessels: costimulation turns up the pressure.Circulation. 2010 Dec 14;122(24):2495-8. doi: 10.1161/CIRCULATIONAHA.110.991059. Epub 2010 Nov 29. Circulation. 2010. PMID: 21126974 Free PMC article. No abstract available.
References
-
- Marvar PJ, Gordon FJ, Harrison DG. Blood pressure control: salt gets under your skin. Nat Med. 2009;15:487–488. - PubMed
-
- Rodriguez-Iturbe B, Quiroz Y, Nava M, Bonet L, Chavez M, Herrera-Acosta J, Johnson RJ, Pons HA. Reduction of renal immune cell infiltration results in blood pressure control in genetically hypertensive rats. Am J Physiol Renal Physiol. 2002;282:F191–F201. - PubMed
-
- Abbas A, Lichtman A. Cellular and molecular immunology. Philadelphia, PA: Elsevier Saunders; 2005.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

