Hetero-oligomerization of neuronal glutamate transporters
- PMID: 21127051
- PMCID: PMC3030394
- DOI: 10.1074/jbc.M110.187492
Hetero-oligomerization of neuronal glutamate transporters
Abstract
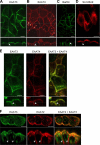
Excitatory amino acid transporters (EAATs) mediate the uptake of glutamate into neuronal and glial cells of the mammalian central nervous system. Two transporters expressed primarily in glia, EAAT1 and EAAT2, are crucial for glutamate homeostasis in the adult mammalian brain. Three neuronal transporters (EAAT3, EAAT4, and EAAT5) appear to have additional functions in regulating and processing cellular excitability. EAATs are assembled as trimers, and the existence of multiple isoforms raises the question of whether certain isoforms can form hetero-oligomers. Co-expression and pulldown experiments of various glutamate transporters showed that EAAT3 and EAAT4, but neither EAAT1 and EAAT2, nor EAAT2 and EAAT3 are capable of co-assembling into heterotrimers. To study the functional consequences of hetero-oligomerization, we co-expressed EAAT3 and the serine-dependent mutant R501C EAAT4 in HEK293 cells and Xenopus laevis oocytes and studied glutamate/serine transport and anion conduction using electrophysiological methods. Individual subunits transport glutamate independently of each other. Apparent substrate affinities are not affected by hetero-oligomerization. However, polarized localization in Madin-Darby canine kidney cells was different for homo- and hetero-oligomers. EAAT3 inserts exclusively into apical membranes of Madin-Darby canine kidney cells when expressed alone. Co-expression with EAAT4 results in additional appearance of basolateral EAAT3. Our results demonstrate the existence of heterotrimeric glutamate transporters and provide novel information about the physiological impact of EAAT oligomerization.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

