Beneficial effects of adenylyl cyclase type 6 (AC6) expression persist using a catalytically inactive AC6 mutant
- PMID: 21127130
- PMCID: PMC3061356
- DOI: 10.1124/mol.110.067298
Beneficial effects of adenylyl cyclase type 6 (AC6) expression persist using a catalytically inactive AC6 mutant
Abstract
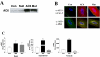
Cardiac-directed expression of AC6 has pronounced favorable effects on cardiac function possibly not linked with cAMP production. To determine rigorously whether cAMP generation is required for the beneficial effects of increased AC6 expression, we generated a catalytically inactive AC6 mutant (AC6mut) that has markedly diminished cAMP generating capacity by replacing aspartic acid with alanine at position 426 in the C1 domain (catalytic region) of AC6. Gene transfer of AC6 or AC6mut (adenovirus-mediated) in adult rat cardiac myocytes resulted in similar expression levels and intracellular distribution, but AC6mut expression was associated with marked reduction in cAMP production. Despite marked reduction in cAMP generation, AC6mut influenced intracellular signaling events similarly to that observed after expression of catalytically intact AC6. For example, both AC6 and AC6mut reduced phenylephrine-induced cardiac myocyte hypertrophy and apoptosis (p < 0.001), expression of cardiac ankyrin repeat protein (p < 0.01), and phospholamban (p < 0.05). AC6mut expression, similar to its catalytically intact cohort, was associated with increased Ca2+ transients in cardiac myocytes after isoproterenol stimulation. Many of the biological effects of AC6 expression are replicated by a catalytically inactive AC6 mutant, indicating that the mechanisms for these effects do not require increased cAMP generation.
Figures
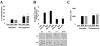
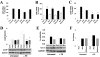
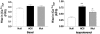

Similar articles
-
Cardiac-directed expression of a catalytically inactive adenylyl cyclase 6 protects the heart from sustained β-adrenergic stimulation.PLoS One. 2017 Aug 2;12(8):e0181282. doi: 10.1371/journal.pone.0181282. eCollection 2017. PLoS One. 2017. PMID: 28767701 Free PMC article.
-
Preserved cardiac function despite marked impairment of cAMP generation.PLoS One. 2013 Sep 16;8(9):e72151. doi: 10.1371/journal.pone.0072151. eCollection 2013. PLoS One. 2013. PMID: 24147149 Free PMC article.
-
Adenylyl cyclase 6 improves calcium uptake and left ventricular function in aged hearts.J Am Coll Cardiol. 2011 May 3;57(18):1846-55. doi: 10.1016/j.jacc.2010.11.052. J Am Coll Cardiol. 2011. PMID: 21527160 Free PMC article.
-
Regulation of phospholamban and troponin-I phosphorylation in the intact rat cardiomyocytes by adrenergic and cholinergic stimuli: roles of cyclic nucleotides, calcium, protein kinases and phosphatases and depolarization.Mol Cell Biochem. 1995 Aug-Sep;149-150:103-26. doi: 10.1007/BF01076569. Mol Cell Biochem. 1995. PMID: 8569720 Review.
-
Unanticipated signaling events associated with cardiac adenylyl cyclase gene transfer.J Mol Cell Cardiol. 2011 May;50(5):751-8. doi: 10.1016/j.yjmcc.2011.02.009. Epub 2011 Feb 23. J Mol Cell Cardiol. 2011. PMID: 21354173 Free PMC article. Review.
Cited by
-
Methods in cardiomyocyte isolation, culture, and gene transfer.J Mol Cell Cardiol. 2011 Sep;51(3):288-98. doi: 10.1016/j.yjmcc.2011.06.012. Epub 2011 Jun 24. J Mol Cell Cardiol. 2011. PMID: 21723873 Free PMC article. Review.
-
Intravenous AAV8 Encoding Urocortin-2 Increases Function of the Failing Heart in Mice.Hum Gene Ther. 2015 Jun;26(6):347-56. doi: 10.1089/hum.2014.157. Epub 2015 Apr 9. Hum Gene Ther. 2015. PMID: 25760560 Free PMC article.
-
Fine Tuning Adenylyl Cyclase as a (Gene) Therapy for Heart Failure.JACC Basic Transl Sci. 2016 Dec 26;1(7):630-632. doi: 10.1016/j.jacbts.2016.10.005. eCollection 2016 Dec. JACC Basic Transl Sci. 2016. PMID: 30167546 Free PMC article.
-
Prospects for gene transfer for clinical heart failure.Gene Ther. 2012 Jun;19(6):606-12. doi: 10.1038/gt.2012.36. Epub 2012 Apr 26. Gene Ther. 2012. PMID: 22534469 Free PMC article. Review.
-
Cyclic AMP synthesis and hydrolysis in the normal and failing heart.Pflugers Arch. 2014 Jun;466(6):1163-75. doi: 10.1007/s00424-014-1515-1. Epub 2014 Apr 24. Pflugers Arch. 2014. PMID: 24756197 Review.
References
-
- Aihara Y, Kurabayashi M, Saito Y, Ohyama Y, Tanaka T, Takeda S, Tomaru K, Sekiguchi K, Arai M, Nakamura T, et al. (2000) Cardiac ankyrin repeat protein is a novel marker of cardiac hypertrophy: role of M-CAT element within the promoter. Hypertension 36:48–53 - PubMed
-
- Bisognano JD, Weinberger HD, Bohlmeyer TJ, Pende A, Raynolds MV, Sastravaha A, Roden R, Asano K, Blaxall BC, Wu SC, et al. (2000) Myocardial-directed overexpression of the human beta(1)-adrenergic receptor in transgenic mice. J Mol Cell Cardiol 32:817–830 - PubMed
-
- Bousette N, Chugh S, Fong V, Isserlin R, Kim KH, Volchuk A, Backx PH, Liu P, Kislinger T, MacLennan DH, et al. (2010) Constitutively active calcineurin induces cardiac endoplasmic reticulum stress and protects against apoptosis that is mediated by alpha-crystallin-B. Proc Natl Acad Sci USA 107:18481–18486 - PMC - PubMed
-
- Brognard J, Sierecki E, Gao T, Newton AC. (2007) PHLPP and a second isoform, PHLPP2, differentially attenuate the amplitude of Akt signaling by regulating distinct Akt isoforms. Mol Cell 25:917–931 - PubMed
-
- Chen-Goodspeed M, Lukan AN, Dessauer CW. (2005) Modeling of Galpha(s) and Galpha(i) regulation of human type V and VI adenylyl cyclase. J Biol Chem 280:1808–1816 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

