ROS and CHOP are critical for dibenzylideneacetone to sensitize tumor cells to TRAIL through induction of death receptors and downregulation of cell survival proteins
- PMID: 21127198
- PMCID: PMC3022089
- DOI: 10.1158/0008-5472.CAN-10-3121
ROS and CHOP are critical for dibenzylideneacetone to sensitize tumor cells to TRAIL through induction of death receptors and downregulation of cell survival proteins
Retraction in
-
Retraction: ROS and CHOP Are Critical for Dibenzylideneacetone to Sensitize Tumor Cells to TRAIL through Induction of Death Receptors and Downregulation of Cell Survival Proteins.Cancer Res. 2018 Sep 1;78(17):5185. doi: 10.1158/0008-5472.CAN-18-2370. Cancer Res. 2018. PMID: 30181309 Free PMC article. No abstract available.
Abstract
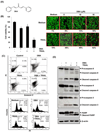
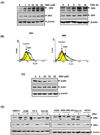
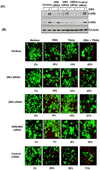
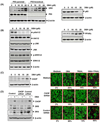
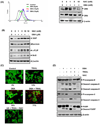
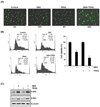
Because tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) selectively kills tumor cells, it is being tested in cancer patients. Unfortunately, patients develop resistance to the cytokine, therefore, agents that can sensitize cells to TRAIL are urgently needed. In this study, we investigated whether dibenzylideneacetone (DBA) can sensitize cancer cells to TRAIL and potentiates TRAIL-induced apoptosis. As indicated by accumulation of the membrane phospholipid phosphatidylserine, DNA breaks, intracellular esterase activity, and activation of caspase-8, -9, and -3, we concluded that DBA potentiated TRAIL-induced apoptosis in colon cancer cells. DBA also converted TRAIL resistant-cells to TRAIL-sensitive. When examined for the mechanism, we found that DBA decreased the expression of antiapoptotic proteins and decoy receptor-2 and increased proapoptotic proteins. DBA also induced both death receptor (DR)-5 and DR4. Knockdown of DR5 and DR4 by small interfering RNA (SiRNA) reduced the sensitizing effect of DBA on TRAIL-induced apoptosis. In addition, DBA increased the expression of CHOP proteins. Knockdown of CHOP by siRNA decreased the induction of DBA-induced DR5 expression and apoptosis. Induction of receptors by DBA, however, was p53-independent, as deletion of p53 had no effect on receptor induction. We observed that DBA-induced induction of DR5 and DR4 was mediated through generation of reactive oxygen species (ROS), as N-acetylcysteine blocked the induction of death receptors and suppression of cell survival proteins by DBA. Overall, our results show that DBA potentiates TRAIL-induced apoptosis through downregulation of cell survival proteins and upregulation of death receptors via activation of ROS and CHOP mediated pathways.
© 2010 AACR.
Figures

Similar articles
-
Gossypol induces death receptor-5 through activation of the ROS-ERK-CHOP pathway and sensitizes colon cancer cells to TRAIL.J Biol Chem. 2010 Nov 12;285(46):35418-27. doi: 10.1074/jbc.M110.172767. Epub 2010 Sep 13. J Biol Chem. 2010. Retraction in: J Biol Chem. 2016 Aug 5;291(32):16923. doi: 10.1074/jbc.A110.172767. PMID: 20837473 Free PMC article. Retracted.
-
Garcinol potentiates TRAIL-induced apoptosis through modulation of death receptors and antiapoptotic proteins.Mol Cancer Ther. 2010 Apr;9(4):856-68. doi: 10.1158/1535-7163.MCT-09-1113. Epub 2010 Apr 6. Mol Cancer Ther. 2010. Retraction in: Mol Cancer Ther. 2018 Sep;17(9):2073. doi: 10.1158/1535-7163.MCT-18-0870. PMID: 20371723 Free PMC article. Retracted.
-
Azadirone, a limonoid tetranortriterpene, induces death receptors and sensitizes human cancer cells to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) through a p53 protein-independent mechanism: evidence for the role of the ROS-ERK-CHOP-death receptor pathway.J Biol Chem. 2013 Nov 8;288(45):32343-32356. doi: 10.1074/jbc.M113.455188. Epub 2013 Sep 27. J Biol Chem. 2013. PMID: 24078627 Free PMC article.
-
Capsazepine, a TRPV1 antagonist, sensitizes colorectal cancer cells to apoptosis by TRAIL through ROS-JNK-CHOP-mediated upregulation of death receptors.Free Radic Biol Med. 2012 Nov 15;53(10):1977-87. doi: 10.1016/j.freeradbiomed.2012.08.012. Epub 2012 Aug 15. Free Radic Biol Med. 2012. PMID: 22922338 Free PMC article.
-
The herbal compound cryptotanshinone restores sensitivity in cancer cells that are resistant to the tumor necrosis factor-related apoptosis-inducing ligand.J Biol Chem. 2013 Oct 11;288(41):29923-33. doi: 10.1074/jbc.M113.483909. Epub 2013 Aug 28. J Biol Chem. 2013. PMID: 23986445 Free PMC article.
Cited by
-
Alternol Sensitizes Renal Carcinoma Cells to TRAIL-Induced Apoptosis.Front Pharmacol. 2021 Mar 25;12:560903. doi: 10.3389/fphar.2021.560903. eCollection 2021. Front Pharmacol. 2021. PMID: 33841136 Free PMC article.
-
Phosphorylated IκBα predicts poor prognosis in activated B-cell lymphoma and its inhibition with thymoquinone induces apoptosis via ROS release.PLoS One. 2013;8(3):e60540. doi: 10.1371/journal.pone.0060540. Epub 2013 Mar 28. PLoS One. 2013. PMID: 23555990 Free PMC article.
-
Trailing TRAIL Resistance: Novel Targets for TRAIL Sensitization in Cancer Cells.Front Oncol. 2015 Apr 2;5:69. doi: 10.3389/fonc.2015.00069. eCollection 2015. Front Oncol. 2015. PMID: 25883904 Free PMC article. Review.
-
ITCH-dependent proteasomal degradation of c-FLIP induced by the anti-HER3 antibody 9F7-F11 promotes DR5/caspase 8-mediated apoptosis of tumor cells.Cell Commun Signal. 2019 Aug 23;17(1):106. doi: 10.1186/s12964-019-0413-8. Cell Commun Signal. 2019. PMID: 31443721 Free PMC article.
-
Analysis of apoptosis methods recently used in Cancer Research and Cell Death & Disease publications.Cell Death Dis. 2012 Feb 2;3(2):e263. doi: 10.1038/cddis.2012.2. Cell Death Dis. 2012. PMID: 22297295 Free PMC article. No abstract available.
References
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. - PubMed
-
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. - PubMed
-
- Plummer R, Attard G, Pacey S, et al. Phase 1 and pharmacokinetic study of lexatumumab in patients with advanced cancers. Clin Cancer Res. 2007;13:6187–6194. - PubMed
-
- Hotte SJ, Hirte HW, Chen EX, et al. A phase 1 study of mapatumumab (fully human monoclonal antibody to TRAIL-R1) in patients with advanced solid malignancies. Clin Cancer Res. 2008;14:3450–3455. - PubMed
-
- Camidge DR, Herbst RS, Gordon MS, et al. A phase I safety and pharmacokinetic study of the death receptor 5 agonistic antibody PRO95780 in patients with advanced malignancies. Clin Cancer Res. 2010;16:1256–1263. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

