Endothelial nitric oxide modulates expression and processing of amyloid precursor protein
- PMID: 21127294
- PMCID: PMC3064266
- DOI: 10.1161/CIRCRESAHA.110.233080
Endothelial nitric oxide modulates expression and processing of amyloid precursor protein
Abstract
Rationale: the exact etiology of sporadic Alzheimer disease (AD) is unclear, but it is interesting that several cardiovascular risk factors are associated with higher incidence of AD. The link between these risk factors and AD has yet to be identified; however, a common feature is endothelial dysfunction, specifically, decreased bioavailability of nitric oxide (NO).
Objective: to determine the relationship between endothelial derived NO and the expression and processing of amyloid precursor protein (APP).
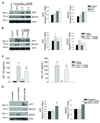
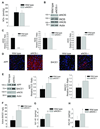
Methods and results: we used human brain microvascular endothelial cells to examine the role of NO in modulating APP expression and processing in vitro. Inhibition of endothelial nitric oxide synthase (eNOS) with the specific NOS inhibitor L-NAME (N(G)-nitro-l-arginine methyl ester) led to increased APP and BACE1 (β-site APP-cleaving enzyme1) protein levels, as well as increased secretion of the amyloidogenic peptide amyloid β (Aβ) (control 10.93 ± 0.70 pg/mL; L-NAME 168.21 ± 27.38 pg/mL; P<0.001). To examine the role of NO in modulation of APP expression and processing in vivo, we used brain and cerebral microvessels from eNOS-deficient (eNOS(-/-)) mice. Brain tissue from eNOS(-/-) mice had statistically higher APP and BACE1 protein levels, as well as increased BACE1 enzyme activity and Aβ (Aβ(1)(-)(42) wild-type control, 0.737 pg/mg; eNOS(-/-), 1.475 pg/mg; P<0.05), compared with wild-type controls (n=6 to 8 animals per background). Brain microvessels from eNOS(-/-) mice also showed statistically higher BACE1 protein levels as compared with wild-type control.
Conclusions: our data suggest that endothelial NO plays an important role in modulating APP expression and processing within the brain and cerebrovasculature. The NO/cGMP pathway may be an important therapeutic target in preventing and treating mild cognitive impairment, as well as AD.
Figures


Comment in
-
No answer to Alzheimer's disease?Circ Res. 2010 Dec 10;107(12):1400-2. doi: 10.1161/CIRCRESAHA.110.234450. Epub 2010 Dec 2. Circ Res. 2010. PMID: 21127292 No abstract available.
References
-
- Gorevic PD, Goni F, Pons-Estel B, Alvarez F, Peress NS, Frangione B. Isolation and partial characterization of neurofibrillary tangles and amyloid plaque core in Alzheimer' disease: immunohistological studies. J Neuropathol Exp Neurol. 1986;45:647–664. - PubMed
-
- Glenner GG, Wong CW. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984;120:885–890. - PubMed
-
- Hussain I, Powell D, Howlett DR, Tew DG, Meek TD, Chapman C, Gloger IS, Murphy KE, Southan CD, Ryan DM, Smith TS, Simmons DL, Walsh FS, Dingwall C, Christie G. Identification of a novel aspartic protease (Asp 2) as beta-secretase. Mol Cell Neurosci. 1999;14:419–427. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

