ΔNp63 is an ectodermal gatekeeper of epidermal morphogenesis
- PMID: 21127502
- PMCID: PMC3131930
- DOI: 10.1038/cdd.2010.159
ΔNp63 is an ectodermal gatekeeper of epidermal morphogenesis
Abstract
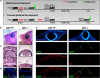
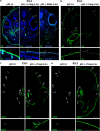
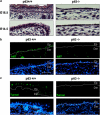
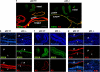
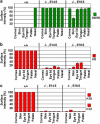
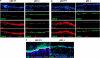
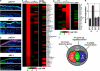
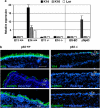
p63, a member of p53 family, has a significant role in the development and maintenance of stratified epithelia. However, a persistent dispute remained over the last decade concerning the interpretation of the severe failure of p63-null embryos to develop stratified epithelia. In this study, by investigating both p63-deficient strains, we demonstrated that p63-deficient epithelia failed to develop beyond ectodermal stage as they remained a monolayer of non-proliferating cells expressing K8/K18. Importantly, in the absence of p63, corneal-epithelial commitment (which occurs at embryonic day 12.5 of mouse embryogenesis) was hampered 3 weeks before corneal stem cell renewal (that begins at P14). Taken together, these data illustrate the significant role of p63 in epithelial embryogenesis, before and independently of other functions of p63 in adult stem cells regulation. Transcriptome analysis of laser captured-embryonic tissues confirmed the latter hypothesis, demonstrating that a battery of epidermal genes that were activated in wild-type epidermis remained silent in p63-null tissues. Furthermore, we defined a subset of novel bona fide p63-induced genes orchestrating first epidermal stratification and a subset of p63-repressed mesodermal-specific genes. These data highlight the earliest recognized action of ΔNp63 in the induction epidermal morphogenesis at E11.5. In the absence of p63, a mesodermal program is activated while epidermal morphogenesis does not initiate.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

