Clinical profile of vigabatrin as monotherapy for treatment of infantile spasms
- PMID: 21127692
- PMCID: PMC2987507
- DOI: 10.2147/NDT.S5235
Clinical profile of vigabatrin as monotherapy for treatment of infantile spasms
Abstract
Vigabatrin, the first therapeutic agent to be approved by the Food and Drug Administration for the treatment of infantile spasms, as well as for adjunctive use in the treatment of refractory complex partial epilepsy, represents an important advance for patients with difficult-to-manage epilepsy. This review summarizes the complex history, chemistry, and pharmacology, as well as the clinical data leading to the approval of vigabatrin for infantile spasms in the US. The long path to its approval reflects the visual system and white matter toxicity concerns with this agent. This review provides a brief description of these concerns, and the regulatory safety monitoring and mitigation systems that have been put in place to enhance benefit over risk.
Keywords: infantile spasms; monotherapy; vigabatrin.
Figures
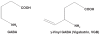
Similar articles
-
Vigabatrin for infantile spasms.Pharmacotherapy. 2011 Mar;31(3):298-311. doi: 10.1592/phco.31.3.298. Pharmacotherapy. 2011. PMID: 21361740 Review.
-
Efficacy and safety of vigabatrin in Japanese patients with infantile spasms: Primary short-term study and extension study.Epilepsy Behav. 2018 Jan;78:134-141. doi: 10.1016/j.yebeh.2017.09.010. Epub 2017 Dec 22. Epilepsy Behav. 2018. PMID: 29190579
-
Primer on visual field testing, electroretinography, and other visual assessments for patients treated with vigabatrin.Acta Neurol Scand Suppl. 2011;(192):48-56. doi: 10.1111/j.1600-0404.2011.01600.x. Acta Neurol Scand Suppl. 2011. PMID: 22061180 Review.
-
A risk-benefit assessment of treatments for infantile spasms.Drug Saf. 2001;24(11):813-28. doi: 10.2165/00002018-200124110-00003. Drug Saf. 2001. PMID: 11665869 Review.
-
Sabril® registry 5-year results: Characteristics of adult patients treated with vigabatrin.Epilepsy Behav. 2016 Mar;56:15-9. doi: 10.1016/j.yebeh.2015.12.004. Epub 2016 Jan 22. Epilepsy Behav. 2016. PMID: 26807550
Cited by
-
CPP-115, a vigabatrin analogue, decreases spasms in the multiple-hit rat model of infantile spasms.Epilepsia. 2014 Jan;55(1):94-102. doi: 10.1111/epi.12424. Epub 2013 Oct 28. Epilepsia. 2014. PMID: 24321005 Free PMC article.
-
West Syndrome: A Review and Guide for Paediatricians.Clin Drug Investig. 2018 Feb;38(2):113-124. doi: 10.1007/s40261-017-0595-z. Clin Drug Investig. 2018. PMID: 29086890 Review.
-
Optimized Treatment for Infantile Spasms: Vigabatrin versus Prednisolone versus Combination Therapy.J Clin Med. 2019 Oct 2;8(10):1591. doi: 10.3390/jcm8101591. J Clin Med. 2019. PMID: 31581698 Free PMC article.
-
An Updated List of Neuromedicinal Plants of Pakistan, Their Uses, and Phytochemistry.Evid Based Complement Alternat Med. 2019 Mar 3;2019:6191505. doi: 10.1155/2019/6191505. eCollection 2019. Evid Based Complement Alternat Med. 2019. PMID: 30941198 Free PMC article. Review.
-
The 2011 E. B. Hershberg award for important discoveries in medicinally active substances: (1S,3S)-3-amino-4-difluoromethylenyl-1-cyclopentanoic acid (CPP-115), a GABA aminotransferase inactivator and new treatment for drug addiction and infantile spasms.J Med Chem. 2012 Jan 26;55(2):567-75. doi: 10.1021/jm201650r. Epub 2012 Jan 10. J Med Chem. 2012. PMID: 22168767 Free PMC article. No abstract available.
References
-
- Sankar R, Derdiarian AT. Vigabatrin. CNS Drug Reviews. 1998;4:260–274.
-
- Krauss GL, Johnson MA, Miller NR. Vigabatrin-associated retinal cone system dysfunction: Electroretinogram and ophthalmologic findings. Neurology. 1998;50:614–618. - PubMed
-
- Butler WH, Ford GP, Newberne JW. A study of the effects of vigabatrin on the central nervous system and retina of Sprague Dawley and Lister-Hooded rats. Toxicol Pathol. 1987;15:143–148. - PubMed

