Delayed conduction and its implications in murine Scn5a(+/-) hearts: independent and interacting effects of genotype, age, and sex
- PMID: 21127902
- PMCID: PMC3016216
- DOI: 10.1007/s00424-010-0906-1
Delayed conduction and its implications in murine Scn5a(+/-) hearts: independent and interacting effects of genotype, age, and sex
Abstract
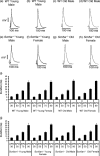
We explored for relationships between SCN5A haploinsufficiency, implicated in clinical arrhythmogenicity, and right ventricular (RV) conduction disorders in Langendorff-perfused, male and female, and young (3 months) and old (>12 month old) Scn5a ( +/-) and wild type (WT) hearts. The investigated conditions of genotype, age, and sex affected latencies but not repolarization time courses of RV monophasic action potentials. This prompted examination of the patterns of RV epicardial activation, its dispersion, and their interrelationships as possible arrhythmic mechanisms using a 64-channel, multi-electrode array. Mean ventricular activation times (T*(MEAN)), spatial dispersions (D* (S)) between recording channels/cardiac cycle, and maximum activation times (T* (MAX)) representing the slowest possible conduction in any given heart were all higher in old male Scn5a ( +/-) compared with young male and old female Scn5a ( +/-) and old male WT. Temporal dispersions (D*(T)) of recording channels were similarly higher in old male Scn5a (+/-) compared with old male WT. All groupings of D*(T), D*(S), and T*(MAX) nevertheless linearly correlated with T*(MEAN), with indistinguishable slopes. The variates explored thus influence D*(T), D*(S), and T*(MAX) through actions on T*(MEAN). These findings in turn correlated with increased levels of fibrosis in young male, young female, and old male Scn5a ( +/-) compared with the corresponding WTs. We thus demonstrate for the first time independent and interacting effects of genotype, age, and sex on epicardial conduction and its dispersions at least partially attributable to fibrotic change, resulting in the greatest effects in old male Scn5a ( +/-) in an absence of alterations in repolarization time courses. This directly implicates altered depolarization in the clinical arrhythmogenicity associated with Scn5a ( +/-).
Figures




References
-
- Alings M, Wilde A. “Brugada” syndrome: clinical data and suggested pathophysiological mechanism. Circulation. 1999;99:666–673. - PubMed
-
- Capulzini L, Brugada P, Brugada J, Brugada R. Arrhythmia and right heart disease: from genetic basis to clinical practice. Rev Esp Cardiol. 2010;63:963–983. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

