Estrogen or estrogen receptor agonist inhibits lipopolysaccharide induced microglial activation and death
- PMID: 21127968
- PMCID: PMC3951894
- DOI: 10.1007/s11064-010-0336-7
Estrogen or estrogen receptor agonist inhibits lipopolysaccharide induced microglial activation and death
Erratum in
- Neurochem Res. 2011 Sep;36(9):1715
Abstract
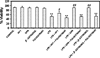
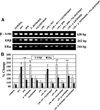
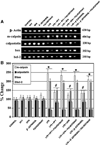
Inflammation is an important pathogenic mechanism in many neurodegenerative disorders. Activated microglia play a pivotal role in releasing pro-inflammatory factors including interleukin-1 (IL-1), tumor necrosis factor-α (TNF-α), and cyclooxygenase-2 (COX-2) for inducing inflammation. While microglia mediated inflammation is essential in maintaining CNS homeostasis, chronic inflammation results in activation of proteases for cell death. Here, we examined the effect of PPT (estrogen receptor α agonist), DPN (estrogen receptor β agonist), and estrogen on rat primary microglia following exposure to lipopolysaccharide (LPS). Exposure of microglia to LPS (200 ng/ml) for 24 h induced cell death. After LPS toxicity for 15 min, microglia were treated with 25 nM PPT, 25 nM DPN, or 100 nM estrogen that prevented cell death by attenuating the release of IL-1α, IL-1β, TNF-α, and COX-2. Treatment of cells with 100 nM fulvestrant (estrogen receptor antagonist) prior to addition of PPT, DPN, or estrogen significantly decreased their ability to prevent cell death, indicating involvement of estrogen receptor (ER) in providing PPT, DPN, or estrogen mediated cytoprotection. Reverse transcriptase polymerase chain reaction (RT-PCR) analyses showed alterations in mRNA expression of Bax, Bcl-2, calpain, and calpastatin during apoptosis. We also examined mRNA expression of ERβ and ERα following exposure of microglia to LPS and subsequent treatment with PPT, DPN, or estrogen. We found that estrogen or estrogen receptor agonists upregulated expression of ERs. Overall, results indicate that estrogen receptor agonist or estrogen uses a receptor mediated pathway to protect microglia from LPS toxicity.
Figures


Similar articles
-
Rat uterine oxytocin receptor and estrogen receptor α and β mRNA levels are regulated by estrogen through multiple estrogen receptors.J Reprod Dev. 2014 Mar 7;60(1):55-61. doi: 10.1262/jrd.2012-139. Epub 2013 Dec 16. J Reprod Dev. 2014. PMID: 24334513 Free PMC article.
-
Estrogen modulates in vitro T cell responses in a concentration- and receptor-dependent manner: effects on intracellular molecular targets and antioxidant enzymes.Mol Immunol. 2013 Dec;56(4):328-39. doi: 10.1016/j.molimm.2013.05.226. Epub 2013 Aug 1. Mol Immunol. 2013. PMID: 23911387
-
Estrogen modulates microglial inflammatory mediator production via interactions with estrogen receptor beta.Endocrinology. 2004 Nov;145(11):5021-32. doi: 10.1210/en.2004-0619. Epub 2004 Jul 15. Endocrinology. 2004. PMID: 15256495
-
Contribution of estrogen receptors alpha and beta to the effects of estradiol in the brain.J Steroid Biochem Mol Biol. 2008 Feb;108(3-5):327-38. doi: 10.1016/j.jsbmb.2007.09.011. Epub 2007 Sep 7. J Steroid Biochem Mol Biol. 2008. PMID: 17936613 Review.
-
No detectable changes in anxiety-related and locomotor behaviors in adult ovariectomized female rats exposed to estradiol, the ERβ agonist DPN or the ERα agonist PPT.Horm Behav. 2023 Jun;152:105363. doi: 10.1016/j.yhbeh.2023.105363. Epub 2023 Apr 21. Horm Behav. 2023. PMID: 37087765 Free PMC article. Review.
Cited by
-
Estrogen receptor alpha deficiency protects against development of cognitive impairment in murine lupus.J Neuroinflammation. 2014 Dec 16;11:171. doi: 10.1186/s12974-014-0171-x. J Neuroinflammation. 2014. PMID: 25510908 Free PMC article.
-
Sex Differences in Affective Dysfunction and Alterations in Parvalbumin in Rodent Models of Early Life Adversity.Front Behav Neurosci. 2021 Nov 4;15:741454. doi: 10.3389/fnbeh.2021.741454. eCollection 2021. Front Behav Neurosci. 2021. PMID: 34803622 Free PMC article. Review.
-
Protective role of endogenous ovarian hormones against learning and memory impairments and brain tissues oxidative damage induced by lipopolysaccharide.Iran Red Crescent Med J. 2014 Mar;16(3):e13954. doi: 10.5812/ircmj.13954. Epub 2014 Mar 5. Iran Red Crescent Med J. 2014. PMID: 24829769 Free PMC article.
-
Administration of low dose estrogen attenuates persistent inflammation, promotes angiogenesis, and improves locomotor function following chronic spinal cord injury in rats.J Neurochem. 2016 May;137(4):604-17. doi: 10.1111/jnc.13610. Epub 2016 Apr 12. J Neurochem. 2016. PMID: 26998684 Free PMC article.
-
Contribution of P2X4 receptor in pain associated with rheumatoid arthritis: a review.Purinergic Signal. 2021 Jun;17(2):201-213. doi: 10.1007/s11302-021-09764-z. Epub 2021 Feb 17. Purinergic Signal. 2021. PMID: 33594635 Free PMC article. Review.
References
-
- Calabrese V, Scapagnini G, Ravagna A, et al. Nitric oxide synthase is present in the cerebrospinal fluid of patients with active multiple sclerosis and is associated with increases in cerebrospinal fluid protein nitrotyrosine and S-nitrosothiols and with changes in glutathione levels. J Neurosci Res. 2002;70:580–587. - PubMed
-
- McGeer PL, McGeer EG. NSAIDs and Alzheimer disease: epidemiological, animal model, and clinical studies. Neurobiol Aging. 2007;28:639–647. - PubMed
-
- Zandi PP, Anthony JC, Hayden KM, et al. Reduced incidence of AD with NSAID but not H2 receptor antagonists: the Cache County Study. Neurology. 2002;59:880–886. - PubMed
-
- Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. - PubMed
-
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

