Design, data management, and population baseline characteristics of the PERFORM magnetic resonance imaging project
- PMID: 21128081
- PMCID: PMC3090565
- DOI: 10.1007/s00415-010-5841-8
Design, data management, and population baseline characteristics of the PERFORM magnetic resonance imaging project
Abstract
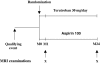
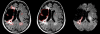
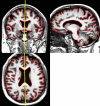
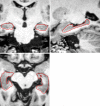
Quantitative information from magnetic resonance imaging (MRI) may substantiate clinical findings and provide additional insight into the mechanism of clinical interventions in therapeutic stroke trials. The PERFORM study is exploring the efficacy of terutroban versus aspirin for secondary prevention in patients with a history of ischemic stroke. We report on the design of an exploratory longitudinal MRI follow-up study that was performed in a subgroup of the PERFORM trial. An international multi-centre longitudinal follow-up MRI study was designed for different MR systems employing safety and efficacy readouts: new T2 lesions, new DWI lesions, whole brain volume change, hippocampal volume change, changes in tissue microstructure as depicted by mean diffusivity and fractional anisotropy, vessel patency on MR angiography, and the presence of and development of new microbleeds. A total of 1,056 patients (men and women ≥ 55 years) were included. The data analysis included 3D reformation, image registration of different contrasts, tissue segmentation, and automated lesion detection. This large international multi-centre study demonstrates how new MRI readouts can be used to provide key information on the evolution of cerebral tissue lesions and within the macrovasculature after atherothrombotic stroke in a large sample of patients.
Figures

Similar articles
-
Results of the PERFORM magnetic resonance imaging study.J Neurol. 2013 Dec;260(12):3071-6. doi: 10.1007/s00415-013-7111-z. J Neurol. 2013. PMID: 24078046 Clinical Trial.
-
Rationale and design of a randomized, double-blind, parallel-group study of terutroban 30 mg/day versus aspirin 100 mg/day in stroke patients: the prevention of cerebrovascular and cardiovascular events of ischemic origin with terutroban in patients with a history of ischemic stroke or transient ischemic attack (PERFORM) study.Cerebrovasc Dis. 2009;27(5):509-18. doi: 10.1159/000212671. Epub 2009 Apr 16. Cerebrovasc Dis. 2009. PMID: 19372653 Clinical Trial.
-
Rationale, design and population baseline characteristics of the PERFORM vascular project: an ancillary study of the Prevention of cerebrovascular and cardiovascular Events of ischemic origin with teRutroban in patients with a history oF ischemic strOke or tRansient ischeMic attack (PERFORM) trial.Cardiovasc Drugs Ther. 2010 Apr;24(2):175-80. doi: 10.1007/s10557-010-6231-2. Cardiovasc Drugs Ther. 2010. PMID: 20490906 Free PMC article. Clinical Trial.
-
Rationale and design of the Prevention of Cerebrovascular and Cardiovascular Events of Ischemic Origin with Terutroban in Patients with a History of Ischemic Stroke or Transient Ischemic Attack (PERFORM) Study.Cerebrovasc Dis. 2009;27 Suppl 3:28-32. doi: 10.1159/000209263. Epub 2009 May 14. Cerebrovasc Dis. 2009. PMID: 19439938 Review.
-
Antiplatelet treatment in primary and secondary stroke prevention in women.Eur J Intern Med. 2012 Oct;23(7):580-5. doi: 10.1016/j.ejim.2012.04.010. Epub 2012 May 12. Eur J Intern Med. 2012. PMID: 22939800 Review.
Cited by
-
Multiple subcortical acute ischemic lesions reflect small vessel disease rather than cardiogenic embolism.J Neurol. 2012 Sep;259(9):1951-7. doi: 10.1007/s00415-012-6451-4. Epub 2012 Feb 17. J Neurol. 2012. PMID: 22349872
-
Results of the PERFORM magnetic resonance imaging study.J Neurol. 2013 Dec;260(12):3071-6. doi: 10.1007/s00415-013-7111-z. J Neurol. 2013. PMID: 24078046 Clinical Trial.
References
-
- Sorbera LA, Serradell N, Bolos J, et al. Terutroban sodium: prostanoid TP receptor antagonist, antithrombotic agent, antiatherosclerotic agent. Drugs Future. 2006;31:867–873. doi: 10.1358/dof.2006.031.10.1038241. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

