Labeled nucleoside triphosphates with reversibly terminating aminoalkoxyl groups
- PMID: 21128174
- PMCID: PMC3858015
- DOI: 10.1080/15257770.2010.536191
Labeled nucleoside triphosphates with reversibly terminating aminoalkoxyl groups
Abstract
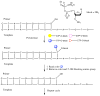
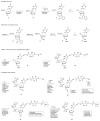
Nucleoside triphosphates having a 3'-ONH₂ blocking group have been prepared with and without fluorescent tags on their nucleobases. DNA polymerases were identified that accepted these, adding a single nucleotide to the 3'-end of a primer in a template-directed extension reaction that then stops. Nitrite chemistry was developed to cleave the 3'-ONH₂ group under mild conditions to allow continued primer extension. Extension-cleavage-extension cycles in solution were demonstrated with untagged nucleotides and mixtures of tagged and untagged nucleotides. Multiple extension-cleavage-extension cycles were demonstrated on an Intelligent Bio-Systems Sequencer, showing the potential of the 3'-ONH₂ blocking group in "next generation sequencing."
Conflict of interest statement
none declared
Figures




References
-
- Metzker ML. Emerging technologies in DNA sequencing. Genome Res. 2005;15:1767–1776. - PubMed
-
- Fuller CW, Middendorf LR, Benner SA, Church GM, Harris T, Huang X, Jovanovich SB, Nelson JR, Schloss JA, Schwartz DC, Vezenov DV. The challenges of sequencing by synthesis. Nature Biotechnology. 2009;27:1013–1023. - PubMed
-
- Hiatt AC. Compositions for enzyme catalyzed template-independent creation of phosphodiester bonds using protected nucleotides. 6232465 US Patent. 2001
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
