Non-steroidal anti-inflammatory drugs: What is the actual risk of liver damage?
- PMID: 21128314
- PMCID: PMC2997980
- DOI: 10.3748/wjg.v16.i45.5651
Non-steroidal anti-inflammatory drugs: What is the actual risk of liver damage?
Abstract
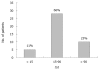
Non-steroidal anti-inflammatory drugs (NSAIDs) constitute a family of drugs, which taken as a group, represents one of the most frequently prescribed around the world. Thus, not surprisingly NSAIDs, along with anti-infectious agents, list on the top for causes of Drug-Induced Liver Injury (DILI). The incidence of liver disease induced by NSAIDs reported in clinical studies is fairly uniform ranging from 0.29/100 000 [95% confidence interval (CI): 0.17-051] to 9/100 000 (95% CI: 6-15). However, compared with these results, a higher risk of liver-related hospitalizations was reported (3-23 per 100 000 patients). NSAIDs exhibit a broad spectrum of liver damage ranging from asymptomatic, transient, hyper-transaminasemia to fulminant hepatic failure. However, under-reporting of asymptomatic, mild cases, as well as of those with transient liver-tests alteration, in conjunction with reports non-compliant with pharmacovigilance criteria to ascertain DILI and flawed epidemiological studies, jeopardize the chance to ascertain the actual risk of NSAIDs hepatotoxicity. Several NSAIDs, namely bromfenac, ibufenac and benoxaprofen, have been withdrawn from the market due to hepatotoxicity; others like nimesulide were never marketed in some countries and withdrawn in others. Indeed, the controversy concerning the actual risk of severe liver disease persists within NSAIDs research. The present work intends (1) to provide a critical analysis of the dissimilar results currently available in the literature concerning the epidemiology of NSAIDS hepatotoxicity; and (2) to review the risk of hepatotoxicity for each one of the most commonly employed compounds of the NSAIDs family, based on past and recently published data.
Figures

References
-
- Andrade RJ, Lucena MI, Fernández MC, Pelaez G, Pachkoria K, García-Ruiz E, García-Muñoz B, González-Grande R, Pizarro A, Durán JA, et al. Drug-induced liver injury: an analysis of 461 incidences submitted to the Spanish registry over a 10-year period. Gastroenterology. 2005;129:512–521. - PubMed
-
- Bjornsson E. Review article: drug-induced liver injury in clinical practice. Aliment Pharmacol Ther. 2010;32:3–13. - PubMed
-
- Hussaini SH, Farrington EA. Idiosyncratic drug-induced liver injury: an overview. Expert Opin Drug Saf. 2007;6:673–684. - PubMed
-
- Fosbøl EL, Gislason GH, Jacobsen S, Folke F, Hansen ML, Schramm TK, Sørensen R, Rasmussen JN, Andersen SS, Abildstrom SZ, et al. Risk of myocardial infarction and death associated with the use of nonsteroidal anti-inflammatory drugs (NSAIDs) among healthy individuals: a nationwide cohort study. Clin Pharmacol Ther. 2009;85:190–197. - PubMed
-
- Drug Safety Update. Volume 2, Issue 7, February 2009.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

