Mitochondria and redox signaling in steatohepatitis
- PMID: 21128703
- PMCID: PMC3118705
- DOI: 10.1089/ars.2010.3795
Mitochondria and redox signaling in steatohepatitis
Abstract
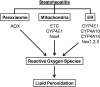
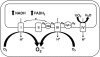
Alcoholic and nonalcoholic fatty liver diseases are potentially pathological conditions that can progress to steatohepatitis, fibrosis, and cirrhosis. These conditions affect millions of people throughout the world in part through poor lifestyle choices of excess alcohol consumption, overnutrition, and lack of regular physical activity. Abnormal mitochondrial and cellular redox homeostasis has been documented in steatohepatitis and results in alterations of multiple redox-sensitive signaling cascades. Ultimately, these changes in signaling lead to altered enzyme function and transcriptional activities of proteins critical to mitochondrial and cellular function. In this article, we review the current hypotheses linking mitochondrial redox state to the overall pathophysiology of alcoholic and nonalcoholic steatohepatitis and briefly discuss the current therapeutic options under investigation.
Figures






Comment in
-
Emerging role of redox dysregulation in alcoholic and nonalcoholic fatty liver disease.Antioxid Redox Signal. 2011 Jul 15;15(2):421-4. doi: 10.1089/ars.2011.3897. Epub 2011 May 25. Antioxid Redox Signal. 2011. PMID: 21254858 Free PMC article. Review.
References
-
- Adams LA. Zein CO. Angulo P. Lindor KD. A pilot trial of pentoxifylline in nonalcoholic steatohepatitis. Am J Gastroenterol. 2004;99:2365–2368. - PubMed
-
- Aithal GP. Thomas JA. Kaye PV. Lawson A. Ryder SD. Spendlove I. Austin AS. Freeman JG. Morgan L. Webber J. Randomized, placebo-controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology. 2008;135:1176–1184. - PubMed
-
- Alnemri ES. Hidden powers of the mitochondria. Nat Cell Biol. 1999;1:E40–E42. - PubMed
-
- Applegate MA. Humphries KM. Szweda LI. Reversible inhibition of alpha-ketoglutarate dehydrogenase by hydrogen peroxide: glutathionylation and protection of lipoic acid. Biochemistry. 2008;47:473–478. - PubMed
-
- Argo CK. Caldwell SH. Epidemiology and natural history of non-alcoholic steatohepatitis. Clin Liver Dis. 2009;13:511–531. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

