Dynamic subcellular localization of isoforms of the folate pathway enzyme serine hydroxymethyltransferase (SHMT) through the erythrocytic cycle of Plasmodium falciparum
- PMID: 21129192
- PMCID: PMC3014972
- DOI: 10.1186/1475-2875-9-351
Dynamic subcellular localization of isoforms of the folate pathway enzyme serine hydroxymethyltransferase (SHMT) through the erythrocytic cycle of Plasmodium falciparum
Abstract
Background: The folate pathway enzyme serine hydroxymethyltransferase (SHMT) converts serine to glycine and 5,10-methylenetetrahydrofolate and is essential for the acquisition of one-carbon units for subsequent transfer reactions. 5,10-methylenetetrahydrofolate is used by thymidylate synthase to convert dUMP to dTMP for DNA synthesis. In Plasmodium falciparum an enzymatically functional SHMT (PfSHMTc) and a related, apparently inactive isoform (PfSHMTm) are found, encoded by different genes. Here, patterns of localization of the two isoforms during the parasite erythrocytic cycle are investigated.
Methods: Polyclonal antibodies were raised to PfSHMTc and PfSHMTm, and, together with specific markers for the mitochondrion and apicoplast, were employed in quantitative confocal fluorescence microscopy of blood-stage parasites.
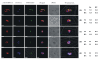
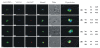
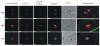
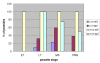
Results: As well as the expected cytoplasmic occupancy of PfSHMTc during all stages, localization into the mitochondrion and apicoplast occurred in a stage-specific manner. Although early trophozoites lacked visible organellar PfSHMTc, a significant percentage of parasites showed such fluorescence during the mid-to-late trophozoite and schizont stages. In the case of the mitochondrion, the majority of parasites in these stages at any given time showed no marked PfSHMTc fluorescence, suggesting that its occupancy of this organelle is of limited duration. PfSHMTm showed a distinctly more pronounced mitochondrial location through most of the erythrocytic cycle and GFP-tagging of its N-terminal region confirmed the predicted presence of a mitochondrial signal sequence. Within the apicoplast, a majority of mitotic schizonts showed a marked concentration of PfSHMTc, whose localization in this organelle was less restricted than for the mitochondrion and persisted from the late trophozoite to the post-mitotic stages. PfSHMTm showed a broadly similar distribution across the cycle, but with a distinctive punctate accumulation towards the ends of elongating apicoplasts. In very late post-mitotic schizonts, both PfSHMTc and PfSHMTm were concentrated in the central region of the parasite that becomes the residual body on erythrocyte lysis and merozoite release.
Conclusions: Both PfSHMTc and PfSHMTm show dynamic, stage-dependent localization among the different compartments of the parasite and sequence analysis suggests they may also reversibly associate with each other, a factor that may be critical to folate cofactor function, given the apparent lack of enzymic activity of PfSHMTm.
Figures







Similar articles
-
Plasmodium serine hydroxymethyltransferase: indispensability and display of distinct localization.Malar J. 2012 Nov 22;11:387. doi: 10.1186/1475-2875-11-387. Malar J. 2012. PMID: 23173711 Free PMC article.
-
Catalytic and ligand-binding characteristics of Plasmodium falciparum serine hydroxymethyltransferase.Mol Biochem Parasitol. 2009 Nov;168(1):74-83. doi: 10.1016/j.molbiopara.2009.06.011. Epub 2009 Jul 8. Mol Biochem Parasitol. 2009. PMID: 19591883 Free PMC article.
-
Stage specific gene expression and cellular localization of two isoforms of the serine hydroxymethyltransferase in the protozoan parasite Leishmania.Mol Biochem Parasitol. 2006 Nov;150(1):63-71. doi: 10.1016/j.molbiopara.2006.06.009. Epub 2006 Jul 13. Mol Biochem Parasitol. 2006. PMID: 16876889
-
A glycine-cleavage complex as part of the folate one-carbon metabolism of Plasmodium falciparum.Trends Parasitol. 2005 Sep;21(9):406-11. doi: 10.1016/j.pt.2005.07.001. Trends Parasitol. 2005. PMID: 16039160 Free PMC article. Review.
-
Plasmodium falciparum: organelle-specific acquisition of lipoic acid.Int J Biochem Cell Biol. 2009 Apr;41(4):748-52. doi: 10.1016/j.biocel.2008.10.028. Epub 2008 Nov 5. Int J Biochem Cell Biol. 2009. PMID: 19027872 Review.
Cited by
-
Plasmodium serine hydroxymethyltransferase: indispensability and display of distinct localization.Malar J. 2012 Nov 22;11:387. doi: 10.1186/1475-2875-11-387. Malar J. 2012. PMID: 23173711 Free PMC article.
-
An ambiguous N-terminus drives the dual targeting of an antioxidant protein Thioredoxin peroxidase (TgTPx1/2) to endosymbiotic organelles in Toxoplasma gondii.PeerJ. 2019 Jul 18;7:e7215. doi: 10.7717/peerj.7215. eCollection 2019. PeerJ. 2019. PMID: 31346496 Free PMC article.
-
Know your enemy: understanding mosquito biology to advance malaria elimination in Africa.Parasitol Res. 2025 Aug 18;124(8):93. doi: 10.1007/s00436-025-08534-9. Parasitol Res. 2025. PMID: 40824459 Free PMC article.
-
Fancy a gene? A surprisingly complex evolutionary history of peroxiredoxins.Microb Cell. 2015 Jan 28;2(2):33-37. doi: 10.15698/mic2015.02.189. Microb Cell. 2015. PMID: 28362003 Free PMC article.
-
Vitamin and cofactor acquisition in apicomplexans: Synthesis versus salvage.J Biol Chem. 2020 Jan 17;295(3):701-714. doi: 10.1074/jbc.AW119.008150. Epub 2019 Nov 25. J Biol Chem. 2020. PMID: 31767680 Free PMC article. Review.
References
-
- Müller IB, Hyde JE. Antimalarial drugs: modes of action and mechanisms of parasite resistance. Future Microbiology. 2010;5:1857–1875. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

