Assembly and dynamics of the bacteriophage T4 homologous recombination machinery
- PMID: 21129202
- PMCID: PMC3016280
- DOI: 10.1186/1743-422X-7-357
Assembly and dynamics of the bacteriophage T4 homologous recombination machinery
Abstract
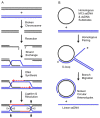
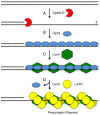
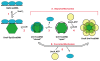
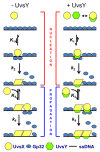
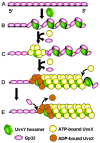
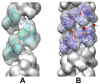
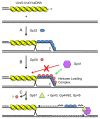
Homologous recombination (HR), a process involving the physical exchange of strands between homologous or nearly homologous DNA molecules, is critical for maintaining the genetic diversity and genome stability of species. Bacteriophage T4 is one of the classic systems for studies of homologous recombination. T4 uses HR for high-frequency genetic exchanges, for homology-directed DNA repair (HDR) processes including DNA double-strand break repair, and for the initiation of DNA replication (RDR). T4 recombination proteins are expressed at high levels during T4 infection in E. coli, and share strong sequence, structural, and/or functional conservation with their counterparts in cellular organisms. Biochemical studies of T4 recombination have provided key insights on DNA strand exchange mechanisms, on the structure and function of recombination proteins, and on the coordination of recombination and DNA synthesis activities during RDR and HDR. Recent years have seen the development of detailed biochemical models for the assembly and dynamics of presynaptic filaments in the T4 recombination system, for the atomic structure of T4 UvsX recombinase, and for the roles of DNA helicases in T4 recombination. The goal of this chapter is to review these recent advances and their implications for HR and HDR mechanisms in all organisms.
Figures
References
-
- Pierce AJ, Stark JM, Araujo FD, Moynahan ME, Berwick M, Jasin M. Double-strand breaks and tumorigenesis. Trends Cell Biol. 2001;11:S52–59. - PubMed
-
- Mosig G. In: Molecular Biology of Bacteriophage T4. Karam JD, editor. ASM Press, Washington, DC; 1994. Homologous recombination; pp. 54–82.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

