Lipoprotein lipase responds similarly to tinzaparin as to conventional heparin during hemodialysis
- PMID: 21129229
- PMCID: PMC3004894
- DOI: 10.1186/1471-2369-11-33
Lipoprotein lipase responds similarly to tinzaparin as to conventional heparin during hemodialysis
Abstract
Background: Low molecular weight (LMW) heparins are used for anticoagulation during hemodialysis (HD). Studies in animals have shown that LMW-heparins release lipoprotein lipase (LPL) as efficiently as unfractionated (UF) heparin, but are less able to retard hepatic uptake of the lipase. This raises a concern that the LPL system may become exhausted by LMW-heparin in patients on HD. We have explored this in the setting of clinical HD.
Methods: Twenty patients on chronic hemodialysis were switched from a primed infusion of UF-heparin to a single bolus of tinzaparin. There were long term follow up of variables for the estimation of dialysis efficacy as well as of the LPL release during dialysis and the subsequent impact on the triglycerides.
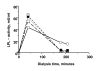
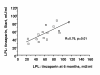
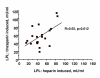
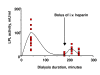
Results: The LPL activity in blood was higher on tinzaparin at 40 but lower at 180 minutes during HD. These values did not change during the 6 month study period. There were significant correlations between the LPL activities in individual patients at the beginning and end of the 6 month study period and between the activities on UF-heparin and on tinzaparin, indicating that tissue LPL was not being exhausted. Triglycerides were higher during the HD-session with tinzaparin than UF-heparin. The plasma lipid/lipoprotein levels did not change during the 6 month study period, nor during a 2-year follow up after the switch from UF-heparin to tinzaparin. Urea reduction rate and Kt/V were reduced by 4 and 7% after 6 months with tinzaparin.
Conclusion: Our data demonstrate that repeated HD with UF-heparin or tinzaparin does not exhaust the LPL-system.
Figures

References
-
- Schmid P, Fischer AG, Wuillemin WA. Low-molecular-weight heparin in patients with renal insufficiency. Swiss Med Wkly. 2009;139(31-32):438–452. - PubMed
-
- Chevreuil O, Hultin M, Ostergaard P, Olivecrona T. Biphasic effects of low-molecular-weight and conventional heparins on chylomicron clearance in rats. Arterioscler Thromb. 1993;13(10):1397–1403. - PubMed
-
- Chevreuil O, Hultin M, Ostergaard P, Olivecrona T. Depletion of lipoprotein lipase after heparin administration. Arterioscler Thromb. 1993;13(10):1391–1396. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

