Novel glucagon-like peptide-1 analog delivered orally reduces postprandial glucose excursions in porcine and canine models
- PMID: 21129350
- PMCID: PMC3005065
- DOI: 10.1177/193229681000400629
Novel glucagon-like peptide-1 analog delivered orally reduces postprandial glucose excursions in porcine and canine models
Abstract
Background: Glucagon-like peptide-1 (GLP-1) and its analogs are associated with a gamut of physiological processes, including induction of insulin release, support of normoglycemia, β-cell function preservation, improved lipid profiles, and increased insulin sensitivity. Thus, GLP-1 harbors significant therapeutic potential for regulating type 2 diabetes mellitus, where its physiological impact is markedly impaired. To date, GLP-1 analogs are only available as injectable dosage forms, and its oral delivery is expected to provide physiological portal/peripheral concentration ratios while fostering patient compliance and adherence.
Methods: Healthy, fasting, enterically cannulated pigs and beagle canines were administered a single dose of the exenatide-based ORMD-0901 formulation 30 min before oral glucose challenges. Blood samples were collected every 15 min for evaluation of ORMD-0901 safety and efficacy in regulating postchallenge glucose excursions.
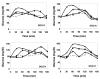
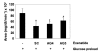
Results: Enterically delivered ORMD-0901 was well tolerated by all animals. ORMD-0901 formulations RG3 and AG2 led to reduced glucose excursions in pigs when delivered prior to a 5 g/kg glucose challenge, where area under the curve (AUC)0-120 values were up to 43% lower than in control sessions. All canines challenged with a glucose load with no prior exposure to exenatide, demonstrated higher AUC0-150 values than in their exenatide-treated sessions. Subcutaneous exenatide delivery amounted to a 51% reduction in mean glucose AUC0-150, while formulations AG4 and AG3 prompted 43% and 29% reductions, respectively.
Conclusions: When delivered enterically, GLP-1 (ORMD-0901) is absorbed from the canine and porcine gastrointestinal tracts and retains its biological activity. Further development of this drug class in an oral dosage form is expected to enhance diabetes control and patient compliance.
© 2010 Diabetes Technology Society.
Figures

References
-
- Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132(6):2131–2157. - PubMed
-
- Vilsbøll T, Holst JJ, Knop FK. The spectrum of antidiabetic actions of GLP-1 in patients with diabetes. Best Pract Res Clin Endocrinol Metab. 2009;23(4):453–462. - PubMed
-
- Kjems LL, Holst JJ, Vølund A, Madsbad S. The influence of GLP-1 on glucose-stimulated insulin secretion: effects on beta-cell sensitivity in type 2 and nondiabetic subjects. Diabetes. 2003;52(2):380–386. - PubMed
-
- Beglinger C, Poller B, Arbit E, Ganzoni C, Gass S, Gomez-Orellana I, Drewe J. Pharmacokinetics and pharmacodynamic effects of oral GLP-1 and PYY3-36: a proof-of-concept study in healthy subjects. Clin Pharmacol Ther. 2008;84(4):468–474. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

