A greater role for the norepinephrine transporter than the serotonin transporter in murine nociception
- PMID: 21129446
- PMCID: PMC3030457
- DOI: 10.1016/j.neuroscience.2010.11.057
A greater role for the norepinephrine transporter than the serotonin transporter in murine nociception
Abstract
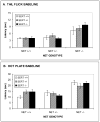
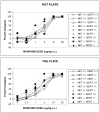
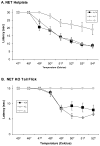
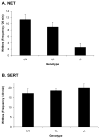
Norepinephrine and serotonin involvement in nociceptive functions is supported by observations of analgesic effects of norepinephrine transporter (NET) and serotonin transporter (SERT) inhibitors such as amitriptyline. However, the relative contribution of NET and SERT to baseline nociception, as well as amitriptyline analgesia, is unclear. Amitriptyline and morphine analgesia in wild-type (WT) mice and littermates with gene knockout (KO) of SERT, NET or both transporters was conducted using the hotplate and tail-flick tests. Hypoalgesia was observed in NET KO mice, and to a lesser extent in SERT KO mice. The magnitude of this hypoalgesia in NET KO mice was so profound that it limited the assessment of drug-induced analgesia. Nonetheless, the necessary exclusion of these subjects because of profound baseline hypoalgesia strongly supports the role of norepinephrine and NET in basal nociceptive behavior while indicating a much smaller role for serotonin and SERT. To further clarify the role of NET and SERT in basal nociceptive sensitivity further experiments were conducted in SERT KO and NET KO mice across a range of temperatures. NET KO mice were again found to have pronounced thermal hypoalgesia compared to WT mice in both the hotplate and tail-flick tests, while only limited effects were observed in SERT KO mice. Furthermore, in the acetic acid writhing test of visceral nociception pronounced hypoalgesia was again found in NET KO mice, but no change in SERT KO mice. As some of these effects may have resulted from developmental consequences of NET KO, the effects of the selective NET blocker nisoxetine and the selective SERT blocker fluoxetine were also examined in WT mice: only nisoxetine produced analgesia in these mice. Collectively these data suggest that NET has a far greater role in determining baseline analgesia, and perhaps other analgesic effects, than SERT in mice.
Published by Elsevier Ltd.
Figures




References
-
- Abdi S, Lee DH, Chung JM. The anti-allodynic effects of amitriptyline, gabapentin, and lidocaine in a rat model of neuropathic pain. Anesth Analg. 1998;87(6):1360–1366. - PubMed
-
- Acton J, McKenna JE, Melzack R. Amitriptyline produces analgesia in the formalin pain test. Exp Neurol. 1992;117(1):94–96. - PubMed
-
- Aimone LD, Jones SL, Gebhart GF. Stimulation-produced descending inhibition from the periaqueductal gray and nucleus raphe magnus in the rat: mediation by spinal monoamines but not opioids. Pain. 1987;31(1):123–136. - PubMed
-
- Ardid D, Eschalier A, Lavarenne J. Evidence for a central but not a peripheral analgesic effect of clomipramine in rats. Pain. 1991;45(1):95–100. - PubMed
-
- Ardid D, Jourdan D, Mestre C, Villanueva L, Le Bars D, Eschalier A. Involvement of bulbospinal pathways in the antinociceptive effect of clomipramine in the rat. Brain Res. 1995;695(2):253–256. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

