Effect of mitoxantrone on outcome of children with first relapse of acute lymphoblastic leukaemia (ALL R3): an open-label randomised trial
- PMID: 21131038
- PMCID: PMC3010035
- DOI: 10.1016/S0140-6736(10)62002-8
Effect of mitoxantrone on outcome of children with first relapse of acute lymphoblastic leukaemia (ALL R3): an open-label randomised trial
Abstract
Background: Although survival of children with acute lymphoblastic leukaemia has improved greatly in the past two decades, the outcome of those who relapse has remained static. We investigated the outcome of children with acute lymphoblastic leukaemia who relapsed on present therapeutic regimens.
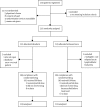
Methods: This open-label randomised trial was undertaken in 22 centres in the UK and Ireland and nine in Australia and New Zealand. Patients aged 1-18 years with first relapse of acute lymphoblastic leukaemia were stratified into high-risk, intermediate-risk, and standard-risk groups on the basis of duration of first complete remission, site of relapse, and immunophenotype. All patients were allocated to receive either idarubicin or mitoxantrone in induction by stratified concealed randomisation. Neither patients nor those giving interventions were masked. After three blocks of therapy, all high-risk group patients and those from the intermediate group with postinduction high minimal residual disease (≥10(-4) cells) received an allogenic stem-cell transplant. Standard-risk and intermediate-risk patients with postinduction low minimal residual disease (<10(-4) cells) continued chemotherapy. The primary outcome was progression-free survival and the method of analysis was intention-to-treat. Randomisation was stopped in December, 2007 because of differences in progression-free and overall survival between the two groups. This trial is registered, reference number ISCRTN45724312.
Findings: Of 239 registered patients, 216 were randomly assigned to either idarubicin (109 analysed) or mitoxantrone (103 analysed). Estimated 3-year progression-free survival was 35·9% (95% CI 25·9-45·9) in the idarubicin group versus 64·6% (54·2-73·2) in the mitoxantrone group (p=0·0004), and 3-year overall survival was 45·2% (34·5-55·3) versus 69·0% (58·5-77·3; p=0·004). Differences in progression-free survival between groups were mainly related to a decrease in disease events (progression, second relapse, disease-related deaths; HR 0·56, 0·34-0·92, p=0·007) rather than an increase in adverse treatment effects (treatment death, second malignancy; HR 0·52, 0·24-1·11, p=0·11).
Interpretation: As compared with idarubicin, mitoxantrone conferred a significant benefit in progression-free and overall survival in children with relapsed acute lymphobastic leukaemia, a potentially useful clinical finding that warrants further investigation.
Funding: Cancer Research UK, Leukaemia and Lymphoma Research, Cancer Council NSW, and Sporting Chance Cancer Foundation.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures



Comment in
-
Mitoxantrone in first-relapse paediatric ALL: the ALL R3 trial.Lancet. 2010 Dec 11;376(9757):1968-70. doi: 10.1016/S0140-6736(10)62194-0. Epub 2010 Dec 3. Lancet. 2010. PMID: 21131040 No abstract available.
-
Mitoxantrone improves the survival of children with first relapse of ALL.Nat Rev Clin Oncol. 2011 Mar;8(3):123. doi: 10.1038/nrclinonc.2011.12. Nat Rev Clin Oncol. 2011. PMID: 21480558 No abstract available.
References
-
- Schrappe M, Nachman J, Hunger S. Educational symposium on long-term results of large prospective clinical trials for childhood acute lymphoblastic leukemia (1985–2000) Leukemia. 2010;24:253–254. - PubMed
-
- Harned TM, Gaynon P. Relapsed acute lymphoblastic leukemia: current status and future opportunities. Curr Oncol Rep. 2008;10:453–458. - PubMed
-
- Lawson SE, Harrison G, Richards S. The UK experience in treating relapsed childhood acute lymphoblastic leukaemia: a report on the medical research council UKALLR1 study. Br J Haematol. 2000;108:531–543. - PubMed
-
- Roy A, Cargill A, Love S. Outcome after first relapse in childhood acute lymphoblastic leukaemia—lessons from the United Kingdom R2 trial. Br J Haematol. 2005;130:67–75. - PubMed
-
- Tallen G, Ratei R, Mann G. Long-term outcome in children with relapsed acute lymphoblastic leukemia after time-point and site-of-relapse stratification and intensified short-course multidrug chemotherapy: results of trial ALL-REZ BFM 90. J Clin Oncol. 2010;28:2339–2347. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

