The kinase inhibitory region of SOCS-1 is sufficient to inhibit T-helper 17 and other immune functions in experimental allergic encephalomyelitis
- PMID: 21131060
- PMCID: PMC3053067
- DOI: 10.1016/j.jneuroim.2010.10.018
The kinase inhibitory region of SOCS-1 is sufficient to inhibit T-helper 17 and other immune functions in experimental allergic encephalomyelitis
Abstract
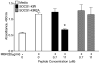
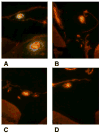
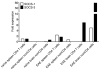
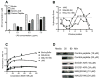
Suppressors of cytokine signaling (SOCS) negatively regulate the immune response, primarily by interfering with the JAK/STAT pathway. We have developed a small peptide corresponding to the kinase inhibitory region (KIR) sequence of SOCS-1, SOCS1-KIR, which inhibits kinase activity by binding to the activation loop of tyrosine kinases such as JAK2 and TYK2. Treatment of SJL/J mice with SOCS1-KIR beginning 12 days post-immunization with myelin basic protein (MBP) resulted in minimal symptoms of EAE, while most control treated mice developed paraplegia. SOCS1-KIR treatment suppressed interleukin-17A (IL-17A) production by MBP-specific lymphocytes, as well as MBP-induced lymphocyte proliferation. When treated with IL-23, a key cytokine in the terminal differentiation of IL-17-producing cells, MBP-sensitized cells produced IL-17A and IFNγ; SOCS1-KIR was able to inhibit the production of these cytokines. SOCS1-KIR also blocked IL-23 and IL-17A activation of STAT3. There is a deficiency of SOCS-1 and SOCS-3 mRNA expression in CD4(+) T cells that infiltrate the CNS, reflecting a deficiency in regulation. Consistent with therapeutic efficacy, SOCS1-KIR reversed the cellular infiltration of the CNS that is associated with EAE. We have shown here that a SOCS-1 like effect can be obtained with a small functional region of the SOCS-1 protein that is easily produced.
Copyright © 2010 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors have no financial conflict of interest.
Figures






References
-
- Abbott NJ, Patabendige AAK, Dolman DEM, Yusof SR, Begey DJ. Structure and function of the blood-brain barrier. Neurobio Dis. 2010;37:13–25. - PubMed
-
- Alexander WS, Hilton DJ. The role of suppressors of cytokine signaling (SOCS) proteins in regulation of the immune response. Ann Rev Immunol. 2004;22:503–529. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

