Using confirmatory factor analysis to evaluate construct validity of the Brief Pain Inventory (BPI)
- PMID: 21131166
- PMCID: PMC3062715
- DOI: 10.1016/j.jpainsymman.2010.05.008
Using confirmatory factor analysis to evaluate construct validity of the Brief Pain Inventory (BPI)
Abstract
Context: The Brief Pain Inventory (BPI) is a frequently used instrument designed to assess the patient-reported outcome of pain. The majority of factor analytic studies have found a two-factor (i.e., pain intensity and pain interference) structure for this instrument; however, because the BPI was developed with an a priori hypothesis of the relationship among its items, it follows that construct validity investigations should use confirmatory factor analysis (CFA).
Objectives: The purpose of this work was to establish the construct validity of the BPI using a CFA framework and demonstrate factorial invariance using a range of demographic variables.
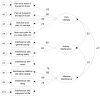
Methods: A retrospective CFA was completed in a sample of individuals diagnosed with HIV/AIDS and cancer (n=364; 63% male; age 21-92 years, M=51.80). A baseline one-factor model was compared against two-factor and three-factor models (i.e., pain intensity, activity interference, and affective interference) that were developed based on the hypothetical design of the instrument.
Results: Fit indices for the three-factor model were statistically superior when compared with the one-factor model and marginally better when compared with the two-factor model. This three-factor structure was found to be invariant across disease, age, and ethnicity groups.
Conclusion: The results of this study provide evidence to support a three-factor representation of the BPI, and the originally hypothesized two-factor structure. Such findings will begin to provide clinical trialists, pharmaceutical sponsors, and regulators with confidence in the psychometric properties of this instrument when considering its inclusion in clinical research.
Copyright © 2011 U.S. Cancer Pain Relief Committee. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures
Similar articles
-
Measurement of affective and activity pain interference using the Brief Pain Inventory (BPI): Cancer and Leukemia Group B 70903.Pain Med. 2012 Nov;13(11):1417-24. doi: 10.1111/j.1526-4637.2012.01498.x. Epub 2012 Oct 30. Pain Med. 2012. PMID: 23110676 Free PMC article. Clinical Trial.
-
The Chinese version of the brief pain inventory-Facial (BPI-F) among different populations: Factor structure and measurement invariance.J Dent. 2023 Dec;139:104771. doi: 10.1016/j.jdent.2023.104771. Epub 2023 Nov 3. J Dent. 2023. PMID: 37925049
-
Validation of the Brief Pain Inventory in Patients With Low Back Pain.Spine (Phila Pa 1976). 2016 Aug 1;41(15):E937-E942. doi: 10.1097/BRS.0000000000001478. Spine (Phila Pa 1976). 2016. PMID: 26839985
-
One, two, or three? Constructs of the brief pain inventory among patients with non-cancer pain in the outpatient setting.J Pain Symptom Manage. 2014 Feb;47(2):325-33. doi: 10.1016/j.jpainsymman.2013.03.023. Epub 2013 Jul 20. J Pain Symptom Manage. 2014. PMID: 23880588
-
Pain assessment: global use of the Brief Pain Inventory.Ann Acad Med Singap. 1994 Mar;23(2):129-38. Ann Acad Med Singap. 1994. PMID: 8080219 Review.
Cited by
-
Minimal Access Surgery for Spinal Metastases: Prospective Evaluation of a Treatment Algorithm Using Patient-Reported Outcomes.World Neurosurg. 2018 Dec;120:e889-e901. doi: 10.1016/j.wneu.2018.08.182. Epub 2018 Sep 4. World Neurosurg. 2018. PMID: 30189298 Free PMC article.
-
Utilization of brief pain inventory as an assessment tool for pain in patients with cancer: a focused review.Indian J Palliat Care. 2011 May;17(2):108-15. doi: 10.4103/0973-1075.84531. Indian J Palliat Care. 2011. PMID: 21976850 Free PMC article.
-
Translation and psychometric properties of the Persian version of the Health of the Nation Outcome Scales for Elderly People (HoNOS65+).Aging Med (Milton). 2021 May 13;4(2):135-145. doi: 10.1002/agm2.12153. eCollection 2021 Jun. Aging Med (Milton). 2021. PMID: 34250432 Free PMC article.
-
Biobehavioral predictors of mood, pain, fatigue, and insomnia in endometrial cancer survivors.Gynecol Oncol. 2024 Dec;191:265-274. doi: 10.1016/j.ygyno.2024.10.024. Epub 2024 Oct 30. Gynecol Oncol. 2024. PMID: 39481346
-
Improving Sleep in People with HIV and Chronic Pain: A Pilot Study of Brief Behavioral Treatment for Insomnia.Behav Sleep Med. 2024 Nov-Dec;22(6):949-959. doi: 10.1080/15402002.2024.2396820. Epub 2024 Sep 8. Behav Sleep Med. 2024. PMID: 39244666
References
-
- Cella D, Nichol MB, Eton D, Nelson JB, Mulani P. Estimating clinically meaningful changes for the Functional Assessment of Cancer Therapy - Prostate: results from a clinical trial of patients with metastatic hormone-refractory prostate cancer. Value Health. 2009;12:124–129. - PubMed
-
- Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. - PubMed
-
- Turk DC, Dworkin RH, McDermott MP, et al. Analyzing multiple endpoints in clinical trials of pain treatments: IMMPACT recommendations. Pain. 2008;139:485–493. - PubMed
-
- US Food and Drug Administration. Guidance for industry. Patient-reported outcome measures: use in medical development to support labeling claims. 2009. [December 9, 2009]. Available from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformati.... - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical