Modeling general proteostasis: proteome balance in health and disease
- PMID: 21131189
- PMCID: PMC3077458
- DOI: 10.1016/j.ceb.2010.11.001
Modeling general proteostasis: proteome balance in health and disease
Abstract
Protein function is generated and maintained by the proteostasis network (PN) (Balch et al. (2008) Science, 319:916). The PN is a modular, yet integrated system unique to each cell type that is sensitive to signaling pathways that direct development and aging, and respond to folding stress. Mismanagement of protein folding and function triggered by genetic, epigenetic and environmental causes poses a major challenge to human health and lifespan. Herein, we address the impact of proteostasis defined by the FoldFx model on our understanding of protein folding and function in biology. FoldFx describes how general proteostasis control (GPC) enables the polypeptide chain sequence to achieve functional balance in the context of the cellular proteome. By linking together the chemical and energetic properties of the protein fold with the composition of the PN we discuss the principle of the proteostasis boundary (PB) as a key component of GPC. The curved surface of the PB observed in 3-dimensional space suggests that the polypeptide chain sequence and the PN operate as an evolutionarily conserved functional unit to generate and sustain protein dynamics required for biology. Modeling general proteostasis provides a rational basis for tackling some of the most challenging diseases facing mankind in the 21st century.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures
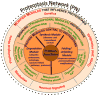


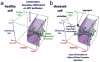
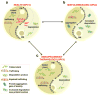
References
-
- Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science. 2008;319:916–919. - PubMed
-
- Powers ET, Morimoto RI, Dillin A, Kelly JW, Balch WE. Biological and chemical approaches to diseases of proteostasis deficiency. Annu Rev Biochem. 2009;78:959–991. This article develops in detail the concept of the FoldFx model for understanding the impact of proteostasis on human biology. - PubMed
-
- Hutt D, Balch WE. Cell Biology. The proteome in balance. Science. 2010;329:766–767. A short perspective on the article by Okiyoneda et al. (2010) (reference 32) that establishes the importance of proteostasis pathways affecting both synthesis and maintenance of the protein fold for function leading to proteome balance. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

