Calsperin is a testis-specific chaperone required for sperm fertility
- PMID: 21131354
- PMCID: PMC3037677
- DOI: 10.1074/jbc.M110.140152
Calsperin is a testis-specific chaperone required for sperm fertility
Abstract
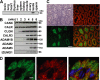
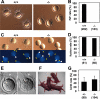
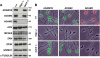
Calnexin (CANX) and calreticulin (CALR) are homologous lectin chaperones located in the endoplasmic reticulum and cooperate to mediate nascent glycoprotein folding. In the testis, calmegin (CLGN) and calsperin (CALR3) are expressed as germ cell-specific counterparts of CANX and CALR, respectively. Here, we show that Calr3(-/-) males produced apparently normal sperm but were infertile because of defective sperm migration from the uterus into the oviduct and defective binding to the zona pellucida. Whereas CLGN was required for ADAM1A/ADAM2 dimerization and subsequent maturation of ADAM3, a sperm membrane protein required for fertilization, we show that CALR3 is a lectin-deficient chaperone directly required for ADAM3 maturation. Our results establish the client specificity of CALR3 and demonstrate that the germ cell-specific CALR-like endoplasmic reticulum chaperones have contrasting functions in the development of male fertility. The identification and understanding of the maturation mechanisms of key sperm proteins will pave the way toward novel approaches for both contraception and treatment of unexplained male infertility.
Figures


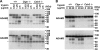
References
-
- Ellgaard L., Helenius A. (2003) Nat. Rev. Mol. Cell Biol. 4, 181–191 - PubMed
-
- Trombetta E. S., Parodi A. J. (2003) Annu. Rev. Cell Dev. Biol. 19, 649–676 - PubMed
-
- Sitia R., Braakman I. (2003) Nature 426, 891–894 - PubMed
-
- Cohen F. E., Kelly J. W. (2003) Nature 426, 905–909 - PubMed
-
- Hammarström P. (2009) FEBS Lett. 583, 2579–2580 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

