A phase I study to determine the safety, pharmacokinetics and pharmacodynamics of a dual VEGFR and FGFR inhibitor, brivanib, in patients with advanced or metastatic solid tumors
- PMID: 21131369
- PMCID: PMC3139984
- DOI: 10.1093/annonc/mdq599
A phase I study to determine the safety, pharmacokinetics and pharmacodynamics of a dual VEGFR and FGFR inhibitor, brivanib, in patients with advanced or metastatic solid tumors
Abstract
Background: This study was designed to determine the safety, pharmacokinetics (PK) and pharmacodynamics (PD) of brivanib in patients with advanced/metastatic solid tumors.
Patients and methods: Ninety patients enrolled in this two-part, phase I open-label study of oral brivanib alaninate. The primary objectives of this study were (in part A) dose-limiting toxicity, maximum tolerated dose (MTD) and the lowest biologically active dose level and (in part B) the optimal dose/dose range. The secondary objectives of this study were preliminary evidence of antitumor activity, PK and PD.
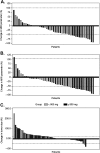
Results: Across part A (open-label dose escalation and MTD) and part B (open-label dose optimization), 68 patients received brivanib alaninate. Brivanib demonstrated a manageable toxicity profile at doses of 180-800 mg. Most toxic effects were mild. Systemic exposure of the active moiety brivanib increased linearly ≤1000 mg/day. The MTD was 800 mg/day. Forty-four patients were treated at the MTD: 20 with 800 mg continuously, 11 with 800 mg intermittently and 13 with 400 mg b.i.d. doses. Partial responses were confirmed in two patients receiving brivanib ≥600 mg. Dynamic contrast-enhanced magnetic resonance imaging demonstrated statistically significant decreases in parameters reflecting tumor vascularity and permeability after multiple doses in the 800-mg continuous q.d. and 400-mg b.i.d. dose cohorts.
Conclusion: In patients with advanced/metastatic cancer, brivanib demonstrates promising antiangiogenic and antitumor activity and manageable toxicity at doses ≤800 mg orally q.d., the recommended phase II study dose.
Trial registration: ClinicalTrials.gov NCT00207051.
Figures
References
-
- Ellis LM, Hicklin DJ. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer. 2008;8(8):579–591. - PubMed
-
- Heath VL, Bicknell R. Anticancer strategies involving the vasculature. Nat Rev Clin Oncol. 2009;6(7):395–404. - PubMed
-
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. - PubMed
-
- Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–2342. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

