TGF-β regulates Nox4, MnSOD and catalase expression, and IL-6 release in airway smooth muscle cells
- PMID: 21131394
- PMCID: PMC3043811
- DOI: 10.1152/ajplung.00134.2010
TGF-β regulates Nox4, MnSOD and catalase expression, and IL-6 release in airway smooth muscle cells
Abstract
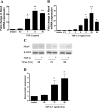
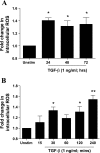
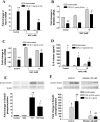
Reactive oxygen species (ROS) are generated as a result of normal cellular metabolism, mainly through the mitochondria and peroxisomes, but their release is enhanced by the activation of oxidant enzymes such as NADPH oxidases or downregulation of endogenous antioxidant enzymes such as manganese-superoxide dismutase (MnSOD) and catalase. Transforming growth factor-β (TGF-β), found to be overexpressed in airway smooth muscle (ASM) from asthmatic and chronic obstructive pulmonary disease patients, may be a pivotal regulator of abnormal ASM cell (ASMC) function in these diseases. An important effect of TGF-β on ASMC inflammatory responses is the induction of IL-6 release. TGF-β also triggers intracellular ROS release in ASMCs by upregulation of NADPH oxidase 4 (Nox4). However, the effect of TGF-β on the expression of key antioxidant enzymes and subsequently on oxidant/antioxidant balance is unknown. Moreover, the role of redox-dependent pathways in the mediation of the proinflammatory effects of TGF-β in ASMCs is unclear. In this study, we show that TGF-β induced the expression of Nox4 while at the same time inhibiting the expression of MnSOD and catalase. This change in oxidant/antioxidant enzymes was accompanied by elevated ROS levels and IL-6 release. Further studies revealed a role for Smad3 and phosphatidyl-inositol kinase-mediated pathways in the induction of oxidant/antioxidant imbalance and IL-6 release. The changes in oxidant/antioxidant enzymes and IL-6 release were reversed by the antioxidants N-acetyl-cysteine (NAC) and ebselen through inhibition of Smad3 phosphorylation, indicating redox-dependent activation of Smad3 by TGF-β. Moreover, these findings suggest a potential role for NAC in preventing TGF-β-mediated pro-oxidant and proinflammatory responses in ASMCs. Knockdown of Nox4 using small interfering RNA partially prevented the inhibition of MnSOD but had no effect on catalase and IL-6 expression. These findings provide novel insights into redox regulation of ASM function by TGF-β.
Figures





References
-
- Ammit AJ, Moir LM, Oliver BG, Hughes JM, Alkhouri H, Ge Q, Burgess JK, Black JL, Roth M. Effect of IL-6 trans-signaling on the pro-remodeling phenotype of airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 292: L199–L206, 2007 - PubMed
-
- Bakin AV, Stourman NV, Sekhar KR, Rinehart C, Yan X, Meredith MJ, Arteaga CL, Freeman ML. Smad3-ATF3 signaling mediates TGF-beta suppression of genes encoding Phase II detoxifying proteins. Free Radic Biol Med 38: 375–387, 2005. - PubMed
-
- Brar SS, Kennedy TP, Whorton AR, Murphy TM, Chitano P, Hoidal JR. Requirement for reactive oxygen species in serum-induced and platelet-derived growth factor-induced growth of airway smooth muscle. J Biol Chem 274: 20017–20026, 1999 - PubMed
-
- Catley MC, Sukkar MB, Chung KF, Jaffee B, Liao SM, Coyle AJ, Haddad el-B, Barnes PJ, Newton R. Validation of the anti-inflammatory properties of small-molecule IkappaB Kinase (IKK)-2 inhibitors by comparison with adenoviral-mediated delivery of dominant-negative IKK1 and IKK2 in human airways smooth muscle. Mol Pharmacol 70: 697–705, 2006 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

