Chronic intermittent hypoxia induces lung growth in adult mice
- PMID: 21131398
- PMCID: PMC3043819
- DOI: 10.1152/ajplung.00239.2010
Chronic intermittent hypoxia induces lung growth in adult mice
Abstract
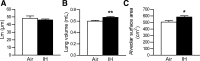
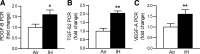
Obstructive sleep apnea (OSA) increases cardiovascular morbidity and mortality, which have been attributed to intermittent hypoxia (IH). The effects of IH on lung structure and function are unknown. We used a mouse model of chronic IH, which mimics the O(2) profile in patients with OSA. We exposed adult C57BL/6J mice to 3 mo of IH with a fraction of inspired oxygen (F(I)(O(2))) nadir of 5% 60 times/h during the 12-h light phase. Control mice were exposed to room air. Lung volumes were measured by quasistatic pressure-volume (PV) curves under anesthesia and by water displacement postmortem. Lungs were processed for morphometry, and the mean airspace chord length (Lm) and alveolar surface area were determined. Lung tissue was stained for markers of proliferation (proliferating cell nuclear antigen), apoptosis (terminal deoxynucleotidyl transferase dUTP nick-end labeling), and type II alveolar epithelial cells (surfactant protein C). Gene microarrays were performed, and results were validated by real-time PCR. IH increased lung volumes by both PV curves (air vs. IH, 1.16 vs. 1.44 ml, P < 0.0001) and water displacement (P < 0.01) without changes in Lm, suggesting that IH increased the alveolar surface area. IH induced a 60% increase in cellular proliferation, but the number of proliferating type II alveolocytes tripled. There was no increase in apoptosis. IH upregulated pathways of cellular movement and cellular growth and development, including key developmental genes vascular endothelial growth factor A and platelet-derived growth factor B. We conclude that IH increases alveolar surface area by stimulating lung growth in adult mice.
Figures






References
-
- Ambalavanan N, Nicola T, Hagood J, Bulger A, Serra R, Murphy-Ullrich J, Oparil S, Chen YF. Transforming growth factor-β signaling mediates hypoxia-induced pulmonary arterial remodeling and inhibition of alveolar development in newborn mouse lung. Am J Physiol Lung Cell Mol Physiol 295: L86–L95, 2008. - PMC - PubMed
-
- Berg JT, Breen EC, Fu Z, Mathieu-Costello O, West JB. Alveolar hypoxia increases gene expression of extracellular matrix proteins and platelet-derived growth factor-B in lung parenchyma. Am J Respir Crit Care Med 158: 1920–1928, 1998. - PubMed
-
- Bixler EO, Vgontzas AN, Lin HM, Ten HT, Rein J, Vela-Bueno A, Kales A. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med 163: 608–613, 2001. - PubMed
-
- Buckley S, Driscoll B, Shi W, Anderson K, Warburton D. Migration and gelatinases in cultured fetal, adult, and hyperoxic alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 281: L427–L434, 2001. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

