Ascorbate and deferoxamine administration after chlorine exposure decrease mortality and lung injury in mice
- PMID: 21131440
- PMCID: PMC3175564
- DOI: 10.1165/rcmb.2010-0432OC
Ascorbate and deferoxamine administration after chlorine exposure decrease mortality and lung injury in mice
Abstract
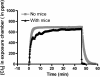
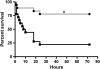
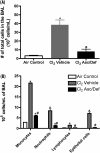
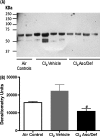
Chlorine (Cl(2)) gas exposure poses an environmental and occupational hazard that frequently results in acute lung injury. There is no effective treatment. We assessed the efficacy of antioxidants, administered after exposure, in decreasing mortality and lung injury in C57BL/6 mice exposed to 600 ppm of Cl(2) for 45 minutes and returned to room air. Ascorbate and deferoxamine were administered intramuscularly every 12 hours and by nose-only inhalation every 24 hours for 3 days starting after 1 hour after exposure. Control mice were exposed to Cl(2) and treated with vehicle (saline or water). Mortality was reduced fourfold in the treatment group compared with the control group (22 versus 78%; P = 0.007). Surviving animals in the treatment group had significantly lower protein concentrations, cell counts, and epithelial cells in their bronchoalveolar lavage (BAL). Lung tissue ascorbate correlated inversely with BAL protein as well as with the number of neutrophils and epithelial cells. In addition, lipid peroxidation was reduced threefold in the BAL of mice treated with ascorbate and deferoxamine when compared with the control group. Administration of ascorbate and deferoxamine reduces mortality and decreases lung injury through reduction of alveolar-capillary permeability, inflammation, and epithelial sloughing and lipid peroxidation.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

