ZO-1 determines adherens and gap junction localization at intercalated disks
- PMID: 21131473
- PMCID: PMC3044061
- DOI: 10.1152/ajpheart.00999.2010
ZO-1 determines adherens and gap junction localization at intercalated disks
Abstract
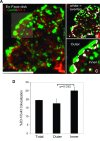
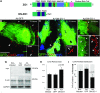
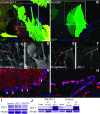
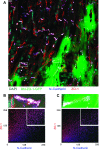
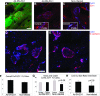
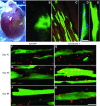
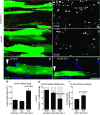
The disruption of the spatial order of electromechanical junctions at myocyte-intercalated disks (ICDs) is a poorly understood characteristic of many cardiac disease states. Here, in vitro and in vivo evidence is provided that zonula occludens-1 (ZO-1) regulates the organization of gap junctions (GJs) and adherens junctions (AJs) at ICDs. We investigated the contribution of ZO-1 to cell-cell junction localization by expressing a dominant-negative ZO-1 construct (DN-ZO-1) in rat ventricular myocytes (VMs). The expression of DN-ZO-1 in cultured neonatal VMs for 72 h reduced the interaction of ZO-1 and N-cadherin, as assayed by colocalization and coimmunoprecipitation, prompting cytoplasmic internalization of AJ and GJ proteins. DN-ZO-1 expression in adult VMs in vivo also reduced N-cadherin colocalization with ZO-1, a phenomenon not observed when the connexin-43 (Cx43)-ZO-1 interaction was disrupted using a mimetic of the ZO-1-binding ligand from Cx43. DN-ZO-1-infected VMs demonstrated large GJs at the ICD periphery and showed a loss of focal ZO-1 concentrations along plaque edges facing the disk interior. Additionally, there was breakdown of the characteristic ICD pattern of small interior and large peripheral GJs. Continuous DN-ZO-1 expression in VMs over postnatal development reduced ICD-associated Cx43 GJs and increased lateralized and cytoplasmic Cx43. We conclude that ZO-1 regulation of GJ localization is via an association with the N-cadherin multiprotein complex and that this is a key determinant of stable localization of both AJs and GJs at the ICD.
Figures
Similar articles
-
Increased association of ZO-1 with connexin43 during remodeling of cardiac gap junctions.Circ Res. 2002 Feb 22;90(3):317-24. doi: 10.1161/hh0302.104471. Circ Res. 2002. PMID: 11861421
-
Increased co-localization of connexin43 and ZO-1 in dissociated adult myocytes.Cell Commun Adhes. 2001;8(4-6):205-8. doi: 10.3109/15419060109080724. Cell Commun Adhes. 2001. PMID: 12064589
-
The organization of adherens junctions and desmosomes at the cardiac intercalated disc is independent of gap junctions.J Cell Sci. 2003 Mar 1;116(Pt 5):875-85. doi: 10.1242/jcs.00258. J Cell Sci. 2003. PMID: 12571285
-
The unstoppable connexin43 carboxyl-terminus: new roles in gap junction organization and wound healing.Ann N Y Acad Sci. 2006 Oct;1080:49-62. doi: 10.1196/annals.1380.005. Ann N Y Acad Sci. 2006. PMID: 17132774 Review.
-
The connexin43 carboxyl terminus and cardiac gap junction organization.Biochim Biophys Acta. 2012 Aug;1818(8):1831-43. doi: 10.1016/j.bbamem.2011.08.006. Epub 2011 Aug 9. Biochim Biophys Acta. 2012. PMID: 21856279 Free PMC article. Review.
Cited by
-
mTOR Regulates Gap Junction Alpha-1 Protein Trafficking in Sertoli Cells and Is Required for the Maintenance of Spermatogenesis in Mice.Biol Reprod. 2016 Jul;95(1):13. doi: 10.1095/biolreprod.115.138016. Epub 2016 Jun 8. Biol Reprod. 2016. PMID: 27281705 Free PMC article.
-
Increased Cardiac Arrhythmogenesis Associated With Gap Junction Remodeling With Upregulation of RNA-Binding Protein FXR1.Circulation. 2018 Feb 6;137(6):605-618. doi: 10.1161/CIRCULATIONAHA.117.028976. Epub 2017 Nov 3. Circulation. 2018. PMID: 29101288 Free PMC article.
-
ZO-1 Regulates Intercalated Disc Composition and Atrioventricular Node Conduction.Circ Res. 2020 Jul 3;127(2):e28-e43. doi: 10.1161/CIRCRESAHA.119.316415. Epub 2020 Apr 29. Circ Res. 2020. PMID: 32347164 Free PMC article.
-
Connexins: Synthesis, Post-Translational Modifications, and Trafficking in Health and Disease.Int J Mol Sci. 2018 Apr 26;19(5):1296. doi: 10.3390/ijms19051296. Int J Mol Sci. 2018. PMID: 29701678 Free PMC article. Review.
-
Icariin Alleviates Bisphenol A Induced Disruption of Intestinal Epithelial Barrier by Maintaining Redox Homeostasis In Vivo and In Vitro.ACS Omega. 2020 Aug 3;5(32):20399-20408. doi: 10.1021/acsomega.0c02364. eCollection 2020 Aug 18. ACS Omega. 2020. Retraction in: ACS Omega. 2021 Jul 19;6(30):20090. doi: 10.1021/acsomega.1c03182. PMID: 32832793 Free PMC article. Retracted.
References
-
- Anderson JM, Fanning AS, Lapierre L, Van Itallie CM. Zonula occludens (ZO)-1 and ZO-2: membrane-associated guanylate kinase homologues (MAGuKs) of the tight junction. Biochem Soc Trans 23: 470–475, 1995. - PubMed
-
- Angst BD, Khan LU, Severs NJ, Whitely K, Rothery S, Thompson RP, Magee AI, Gourdie RG. Dissociated spatial patterning of gap junctions and cell adhesion junctions during postnatal differentiation of ventricular myocardium. Circ Res 80: 88–94, 1997. - PubMed
-
- Barker RJ, Price RL, Gourdie RG. Increased association of ZO-1 with connexin43 during remodeling of cardiac gap junctions. Circ Res 90: 317–324, 2002. - PubMed
-
- Christensen G, Minamisawa S, Gruber PJ, Wang Y, Chien KR. High-efficiency, long-term cardiac expression of foreign genes in living mouse embryos and neonates. Circulation 101: 178–184, 2000. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

