Quantification of calsequestrin 2 (CSQ2) in sheep cardiac muscle and Ca2+-binding protein changes in CSQ2 knockout mice
- PMID: 21131479
- PMCID: PMC3044055
- DOI: 10.1152/ajpheart.00902.2010
Quantification of calsequestrin 2 (CSQ2) in sheep cardiac muscle and Ca2+-binding protein changes in CSQ2 knockout mice
Abstract
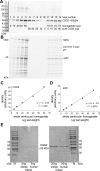
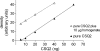
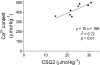
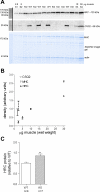
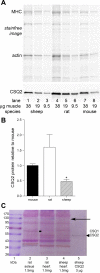
Calsequestrin 2 (CSQ2) is generally regarded as the primary Ca2+-buffering molecule present inside the sarcoplasmic reticulum (SR) in cardiac cells, but findings from CSQ2 knockout experiments raise major questions about its role and necessity. This study determined the absolute amount of CSQ2 present in cardiac ventricular muscle to gauge its likely influence on SR free Ca2+ concentration ([Ca2+]) and maximal Ca2+ capacity. Ventricular tissue from hearts of freshly killed sheep was examined by SDS-PAGE without any fractionation, and CSQ2 was detected by Western blotting; this method avoided the >90% loss of CSQ2 occurring with usual fractionation procedures. Band intensities were compared against those for purified CSQ2 run on the same blots. Fidelity of quantification was verified by demonstrating that CSQ2 added to homogenates was detected with equal efficacy as purified CSQ2 alone. Ventricular tissue from sheep (n=8) contained 24±2 μmol CSQ2/kg wet wt. Total Ca2+ content of the ventricular tissue, measured by atomic absorption spectroscopy, was 430±20 μmol/kg (with SR Ca2+ likely<250 μmol/kg) and displayed a linear correlation with CSQ2 content, with gradient of ∼10 Ca2+ per CSQ2. The large amount of CSQ2 bestows the SR with a high theoretical maximal Ca2+-binding capacity (∼1 mmol Ca2+/kg ventricular tissue, assuming a maximum of ∼40 Ca2+ per CSQ2) and would keep free [Ca2+] within the SR relatively low, energetically favoring Ca2+ uptake and reducing SR leak. In mice with CSQ2 ablated, histidine-rich Ca2+-binding protein was upregulated ∼35% in ventricular tissue, possibly in compensation.
Figures

Similar articles
-
Functional interaction between calsequestrin and ryanodine receptor in the heart.Cell Mol Life Sci. 2013 Aug;70(16):2935-45. doi: 10.1007/s00018-012-1199-7. Epub 2012 Oct 30. Cell Mol Life Sci. 2013. PMID: 23109100 Free PMC article. Review.
-
Characterization of Ca(2+)-Dependent Protein-Protein Interactions within the Ca(2+) Release Units of Cardiac Sarcoplasmic Reticulum.Mol Cells. 2016 Feb;39(2):149-55. doi: 10.14348/molcells.2016.2284. Epub 2015 Dec 15. Mol Cells. 2016. PMID: 26674963 Free PMC article.
-
Calsequestrin content and SERCA determine normal and maximal Ca2+ storage levels in sarcoplasmic reticulum of fast- and slow-twitch fibres of rat.J Physiol. 2009 Jan 15;587(2):443-60. doi: 10.1113/jphysiol.2008.163162. Epub 2008 Nov 24. J Physiol. 2009. PMID: 19029185 Free PMC article.
-
Calsequestrin accumulation in rough endoplasmic reticulum promotes perinuclear Ca2+ release.J Biol Chem. 2012 May 11;287(20):16670-80. doi: 10.1074/jbc.M112.340927. Epub 2012 Mar 28. J Biol Chem. 2012. PMID: 22457350 Free PMC article.
-
Calsequestrin and the calcium release channel of skeletal and cardiac muscle.Prog Biophys Mol Biol. 2004 May;85(1):33-69. doi: 10.1016/j.pbiomolbio.2003.07.001. Prog Biophys Mol Biol. 2004. PMID: 15050380 Review.
Cited by
-
Control of sarcoplasmic reticulum Ca2+ release by stochastic RyR gating within a 3D model of the cardiac dyad and importance of induction decay for CICR termination.Biophys J. 2013 May 21;104(10):2149-59. doi: 10.1016/j.bpj.2013.03.058. Biophys J. 2013. PMID: 23708355 Free PMC article.
-
Important considerations for protein analyses using antibody based techniques: down-sizing Western blotting up-sizes outcomes.J Physiol. 2013 Dec 1;591(23):5823-31. doi: 10.1113/jphysiol.2013.263251. Epub 2013 Oct 14. J Physiol. 2013. PMID: 24127618 Free PMC article.
-
Functional interaction between calsequestrin and ryanodine receptor in the heart.Cell Mol Life Sci. 2013 Aug;70(16):2935-45. doi: 10.1007/s00018-012-1199-7. Epub 2012 Oct 30. Cell Mol Life Sci. 2013. PMID: 23109100 Free PMC article. Review.
-
Controversies in TWEAK-Fn14 signaling in skeletal muscle atrophy and regeneration.Cell Mol Life Sci. 2020 Sep;77(17):3369-3381. doi: 10.1007/s00018-020-03495-x. Epub 2020 Mar 21. Cell Mol Life Sci. 2020. PMID: 32200423 Free PMC article. Review.
-
Conditional ablation and conditional rescue models for Casq2 elucidate the role of development and of cell-type specific expression of Casq2 in the CPVT2 phenotype.Hum Mol Genet. 2018 May 1;27(9):1533-1544. doi: 10.1093/hmg/ddy060. Hum Mol Genet. 2018. PMID: 29452352 Free PMC article.
References
-
- Allen BG, Katz S. Calreticulin and calsequestrin are differentially distributed in canine heart. J Mol Cell Cardiol 32: 2379–2384, 2000 - PubMed
-
- Beard NA, Laver DR, Dulhunty AF. Calsequestrin and the calcium release channel of skeletal and cardiac muscle. Prog Biophys Mol Biol 85: 33–69, 2004 - PubMed
-
- Bers D. Excitation-Contraction Coupling and Cardiac Contractile Force. The Netherlands: Kluwer, 2001
-
- Bers DM, Langer GA. Uncoupling cation effects on cardiac contractility and sarcolemmal Ca2+ binding. Am J Physiol Heart Circ Physiol 237: H332–H341, 1979 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

