Phorbol ester and endothelin-1 alter functional expression of Na+/Ca2+ exchange, K+, and Ca2+ currents in cultured neonatal rat myocytes
- PMID: 21131481
- PMCID: PMC3044063
- DOI: 10.1152/ajpheart.00388.2010
Phorbol ester and endothelin-1 alter functional expression of Na+/Ca2+ exchange, K+, and Ca2+ currents in cultured neonatal rat myocytes
Abstract
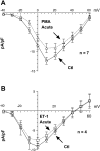
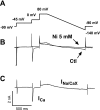
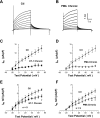
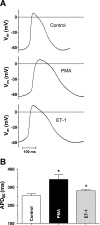
Endothelin-1 (ET-1) and activation of protein kinase C (PKC) have been implicated in alterations of myocyte function in cardiac hypertrophy and heart failure. Changes in cellular Ca2+ handling and electrophysiological properties also occur in these states and may contribute to mechanical dysfunction and arrhythmias. While ET-1 or PKC stimulation induces cellular hypertrophy in cultured neonatal rat ventricular myocytes (NRVMs), a system widely used in studies of hypertrophic signaling, there is little data about electrophysiological changes. Here we studied the effects of ET-1 (100 nM) or the PKC activator phorbol 12-myristate 13-acetate (PMA, 1 μM) on ionic currents in NRVMs. The acute effects of PMA or ET-1 (≤30 min) were small or insignificant. However, PMA or ET-1 exposure for 48-72 h increased cell capacitance by 100 or 25%, respectively, indicating cellular hypertrophy. ET-1 also slightly increased Ca2+ current density (T and L type). Na+/Ca2+ exchange current was increased by chronic pretreatment with either PMA or ET-1. In contrast, transient outward and delayed rectifier K+ currents were strongly downregulated by PMA or ET-1 pretreatment. Inward rectifier K+ current tended toward a decrease at larger negative potential, but time-independent outward K+ current was unaltered by either treatment. The enhanced inward and reduced outward currents also result in action potential prolongation after PMA or ET-1 pretreatment. We conclude that chronic PMA or ET-1 exposure in cultured NRVMs causes altered functional expression of cardiac ion currents, which mimic electrophysiological changes seen in whole animal and human hypertrophy and heart failure.
Figures

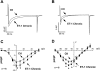
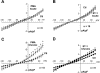


Similar articles
-
[Inhibitory effects of rosiglitazone against endothelin-1-induced proliferation of rat cardiac myocytes: the role of PKC-c-fos pathway].Nan Fang Yi Ke Da Xue Xue Bao. 2008 Jun;28(6):1056-60. Nan Fang Yi Ke Da Xue Xue Bao. 2008. PMID: 18583263 Chinese.
-
Regulation by endothelin-1 of Na+-Ca2+ exchange current (I(NaCa)) from guinea-pig isolated ventricular myocytes.Cell Calcium. 2001 Nov;30(5):351-60. doi: 10.1054/ceca.2001.0244. Cell Calcium. 2001. PMID: 11733942
-
Mesenteric lymph from rats with thermal injury prolongs the action potential and increases Ca2+ transient in rat ventricular myocytes.Shock. 2003 Nov;20(5):458-64. doi: 10.1097/01.shk.0000090602.26659.5c. Shock. 2003. PMID: 14560111
-
Modulation of outward potassium currents in aligned cultures of neonatal rat ventricular myocytes during phorbol ester-induced hypertrophy.J Mol Cell Cardiol. 2001 Jun;33(6):1233-47. doi: 10.1006/jmcc.2001.1386. J Mol Cell Cardiol. 2001. PMID: 11444926
-
Na(+)influx via Na(+)/H(+)exchange activates protein kinase C isozymes delta and epsilon in cultured neonatal rat cardiac myocytes.J Mol Cell Cardiol. 1999 Aug;31(8):1559-72. doi: 10.1006/jmcc.1999.0993. J Mol Cell Cardiol. 1999. PMID: 10423353
Cited by
-
PKCβ/NF-κB pathway in diabetic atrial remodeling.J Physiol Biochem. 2020 Nov;76(4):637-653. doi: 10.1007/s13105-020-00769-7. Epub 2020 Oct 21. J Physiol Biochem. 2020. PMID: 33085045
-
The Na+/Ca²+ exchanger in cardiac ischemia/reperfusion injury.Med Sci Monit. 2012 Nov;18(11):RA161-5. doi: 10.12659/msm.883533. Med Sci Monit. 2012. PMID: 23111750 Free PMC article. Review.
-
Metformin regulates atrial SK2 and SK3 expression through inhibiting the PKC/ERK signaling pathway in type 2 diabetic rats.BMC Cardiovasc Disord. 2018 Dec 13;18(1):236. doi: 10.1186/s12872-018-0950-x. BMC Cardiovasc Disord. 2018. PMID: 30545309 Free PMC article.
-
Calcium and IP3 dynamics in cardiac myocytes: experimental and computational perspectives and approaches.Front Pharmacol. 2014 Mar 6;5:35. doi: 10.3389/fphar.2014.00035. eCollection 2014. Front Pharmacol. 2014. PMID: 24639654 Free PMC article. Review.
-
Oxidative Stress-Induced Afterdepolarizations and Protein Kinase C Signaling.Int J Mol Sci. 2017 Mar 30;18(4):688. doi: 10.3390/ijms18040688. Int J Mol Sci. 2017. PMID: 28358314 Free PMC article.
References
-
- Arai M, Alpert NR, MacLennan DH, Barton P, Periasamy M. Alterations in sarcoplasmic reticulum gene expression in human heart failure. A possible mechanism for alterations in systolic and diastolic properties of the failing myocardium. Circ Res 72: 463–469, 1993 - PubMed
-
- Belin RJ, Sumandea MP, Allen EJ, Schoenfelt K, Wang H, Solaro RJ, de Tombe PP. Augmented protein kinase C-alpha-induced myofilament protein phosphorylation contributes to myofilament dysfunction in experimental congestive heart failure. Circ Res 101: 195–204, 2007 - PubMed
-
- Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol 70: 23–49, 2008 - PubMed
-
- Bers DM. Excitation Contraction Coupling and Cardiac Contractile Force. Dordrecht: Kluwer Academic, 2001
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

