Flavobacterium johnsoniae sprB is part of an operon spanning the additional gliding motility genes sprC, sprD, and sprF
- PMID: 21131497
- PMCID: PMC3021240
- DOI: 10.1128/JB.01203-10
Flavobacterium johnsoniae sprB is part of an operon spanning the additional gliding motility genes sprC, sprD, and sprF
Abstract
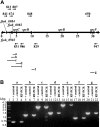
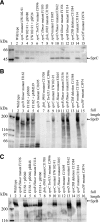
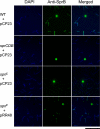
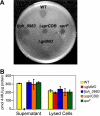
Cells of Flavobacterium johnsoniae move rapidly over surfaces by a process known as gliding motility. Gld proteins are thought to comprise the gliding motor that propels cell surface adhesins, such as the 669-kDa SprB. A novel protein secretion apparatus called the Por secretion system (PorSS) is required for assembly of SprB on the cell surface. Genetic and molecular analyses revealed that sprB is part of a seven-gene operon spanning 29.3 kbp of DNA. In addition to sprB, three other genes of this operon (sprC, sprD, and sprF) are involved in gliding. Mutations in sprB, sprC, sprD, and sprF resulted in cells that failed to form spreading colonies on agar but that exhibited some motility on glass in wet mounts. SprF exhibits some similarity to Porphyromonas gingivalis PorP, which is required for secretion of gingipain protease virulence factors via the P. gingivalis PorSS. F. johnsoniae sprF mutants produced SprB protein but were defective in localization of SprB to the cell surface, suggesting a role for SprF in secretion of SprB. The F. johnsoniae PorSS is involved in secretion of extracellular chitinase in addition to its role in secretion of SprB. SprF was not needed for chitinase secretion and may be specifically required for SprB secretion by the PorSS. Cells with nonpolar mutations in sprC or sprD produced and secreted SprB and propelled it rapidly along the cell surface. Multiple paralogs of sprB, sprC, sprD, and sprF are present in the genome, which may explain why mutations in sprB, sprC, sprD, and sprF do not result in complete loss of motility and suggests the possibility that semiredundant SprB-like adhesins may allow movement of cells over different surfaces.
Figures




Similar articles
-
Flavobacterium johnsoniae GldK, GldL, GldM, and SprA are required for secretion of the cell surface gliding motility adhesins SprB and RemA.J Bacteriol. 2013 Jul;195(14):3201-12. doi: 10.1128/JB.00333-13. Epub 2013 May 10. J Bacteriol. 2013. PMID: 23667240 Free PMC article.
-
The Carboxy-Terminal Region of Flavobacterium johnsoniae SprB Facilitates Its Secretion by the Type IX Secretion System and Propulsion by the Gliding Motility Machinery.J Bacteriol. 2019 Sep 6;201(19):e00218-19. doi: 10.1128/JB.00218-19. Print 2019 Oct 1. J Bacteriol. 2019. PMID: 31262839 Free PMC article.
-
Development and use of a gene deletion strategy for Flavobacterium johnsoniae to identify the redundant gliding motility genes remF, remG, remH, and remI.J Bacteriol. 2011 May;193(10):2418-28. doi: 10.1128/JB.00117-11. Epub 2011 Mar 18. J Bacteriol. 2011. PMID: 21421754 Free PMC article.
-
Flavobacterium gliding motility and the type IX secretion system.Curr Opin Microbiol. 2015 Dec;28:72-7. doi: 10.1016/j.mib.2015.07.016. Epub 2015 Oct 23. Curr Opin Microbiol. 2015. PMID: 26461123 Review.
-
Cytophaga-flavobacterium gliding motility.J Mol Microbiol Biotechnol. 2004;7(1-2):63-71. doi: 10.1159/000077870. J Mol Microbiol Biotechnol. 2004. PMID: 15170404 Review.
Cited by
-
Filamentous structures in the cell envelope are associated with bacteroidetes gliding machinery.Commun Biol. 2023 Jan 23;6(1):94. doi: 10.1038/s42003-023-04472-3. Commun Biol. 2023. PMID: 36690840 Free PMC article.
-
Identification of the Type IX Secretion System Component, PorV (CHU_3238), Involved in Secretion and Localization of Proteins in Cytophaga hutchinsonii.Front Microbiol. 2021 Oct 20;12:742673. doi: 10.3389/fmicb.2021.742673. eCollection 2021. Front Microbiol. 2021. PMID: 34745042 Free PMC article.
-
Flavobacterium johnsoniae GldK, GldL, GldM, and SprA are required for secretion of the cell surface gliding motility adhesins SprB and RemA.J Bacteriol. 2013 Jul;195(14):3201-12. doi: 10.1128/JB.00333-13. Epub 2013 May 10. J Bacteriol. 2013. PMID: 23667240 Free PMC article.
-
Complete genome sequence of the filamentous gliding predatory bacterium Herpetosiphon aurantiacus type strain (114-95(T)).Stand Genomic Sci. 2011 Dec 31;5(3):356-70. doi: 10.4056/sigs.2194987. Epub 2011 Dec 23. Stand Genomic Sci. 2011. PMID: 22675585 Free PMC article.
-
Evidence that the stress hormone cortisol regulates biofilm formation differently among Flavobacterium columnare isolates.Vet Res. 2019 Apr 11;50(1):24. doi: 10.1186/s13567-019-0641-3. Vet Res. 2019. PMID: 30971289 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

