Microfluidic cell culture and its application in high-throughput drug screening: cardiotoxicity assay for hERG channels
- PMID: 21131594
- PMCID: PMC3052261
- DOI: 10.1177/1087057110386218
Microfluidic cell culture and its application in high-throughput drug screening: cardiotoxicity assay for hERG channels
Abstract
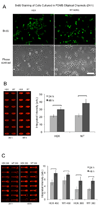
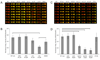
Evaluation of drug cardiotoxicity is essential to the safe development of novel pharmaceuticals. Assessing a compound's risk for prolongation of the surface electrocardiographic QT interval and hence risk for life-threatening arrhythmias is mandated before approval of nearly all new pharmaceuticals. QT prolongation has most commonly been associated with loss of current through hERG (human ether-a-go-go related gene) potassium ion channels due to direct block of the ion channel by drugs or occasionally by inhibition of the plasma membrane expression of the channel protein. To develop an efficient, reliable, and cost-effective hERG screening assay for detecting drug-mediated disruption of hERG membrane trafficking, the authors demonstrate the use of microfluidic-based systems to improve throughput and lower cost of current methods. They validate their microfluidics array platform in polystyrene (PS), cyclo-olefin polymer (COP), and polydimethylsiloxane (PDMS) microchannels for drug-induced disruption of hERG trafficking by culturing stably transfected HEK cells that overexpressed hERG (WT-hERG) and studying their morphology, proliferation rates, hERG protein expression, and response to drug treatment. Results show that WT-hERG cells readily proliferate in PS, COP, and PDMS microfluidic channels. The authors demonstrated that conventional Western blot analysis was possible using cell lysate extracted from a single microchannel. The Western blot analysis also provided important evidence that WT-hERG cells cultured in microchannels maintained regular (well plate-based) expression of hERG. The authors further show that experimental procedures can be streamlined by using direct in-channel immunofluorescence staining in conjunction with detection using an infrared scanner. Finally, treatment of WT-hERG cells with 5 different drugs suggests that PS (and COP) microchannels were more suitable than PDMS microchannels for drug screening applications, particularly for tests involving hydrophobic drug molecules.
Figures




Similar articles
-
Progesterone impairs human ether-a-go-go-related gene (HERG) trafficking by disruption of intracellular cholesterol homeostasis.J Biol Chem. 2011 Jun 24;286(25):22186-94. doi: 10.1074/jbc.M110.198853. Epub 2011 Apr 27. J Biol Chem. 2011. PMID: 21525004 Free PMC article.
-
Propofol inhibits hERG K+ channels and enhances the inhibition effects on its mutations in HEK293 cells.Eur J Pharmacol. 2016 Nov 15;791:168-178. doi: 10.1016/j.ejphar.2016.08.028. Epub 2016 Aug 26. Eur J Pharmacol. 2016. PMID: 27575519
-
Identification of human Ether-à-go-go related gene modulators by three screening platforms in an academic drug-discovery setting.Assay Drug Dev Technol. 2010 Dec;8(6):727-42. doi: 10.1089/adt.2010.0331. Assay Drug Dev Technol. 2010. PMID: 21158687 Free PMC article.
-
The cardiac hERG/IKr potassium channel as pharmacological target: structure, function, regulation, and clinical applications.Curr Pharm Des. 2006;12(18):2271-83. doi: 10.2174/138161206777585102. Curr Pharm Des. 2006. PMID: 16787254 Review.
-
Recent developments in computational prediction of HERG blockage.Curr Top Med Chem. 2013;13(11):1317-26. doi: 10.2174/15680266113139990036. Curr Top Med Chem. 2013. PMID: 23675938 Review.
Cited by
-
Machine learning study of the extended drug-target interaction network informed by pain related voltage-gated sodium channels.Pain. 2024 Apr 1;165(4):908-921. doi: 10.1097/j.pain.0000000000003089. Epub 2023 Oct 18. Pain. 2024. PMID: 37851391 Free PMC article.
-
Application of microfluidic chip technology in pharmaceutical analysis: A review.J Pharm Anal. 2019 Aug;9(4):238-247. doi: 10.1016/j.jpha.2018.12.001. Epub 2018 Dec 6. J Pharm Anal. 2019. PMID: 31452961 Free PMC article.
-
In vitro models to study natural killer cell dynamics in the tumor microenvironment.Front Immunol. 2023 Jun 28;14:1135148. doi: 10.3389/fimmu.2023.1135148. eCollection 2023. Front Immunol. 2023. PMID: 37457703 Free PMC article. Review.
-
Organoid-on-a-chip: Current challenges, trends, and future scope toward medicine.Biomicrofluidics. 2023 Oct 27;17(5):051505. doi: 10.1063/5.0171350. eCollection 2023 Sep. Biomicrofluidics. 2023. PMID: 37900053 Free PMC article. Review.
-
Engineered human pluripotent stem cell-derived cardiac cells and tissues for electrophysiological studies.Drug Discov Today Dis Models. 2012 Winter;9(4):e209-e217. doi: 10.1016/j.ddmod.2012.06.002. Drug Discov Today Dis Models. 2012. PMID: 29422934 Free PMC article.
References
-
- Kongsamut S, Kang J, Chen X, Roehr J, Rampe D. A comparison of the receptor binding and HERG channel affinities for a series of antipsychotic drugs. European Journal of Pharmacology. 2002;450:37–41. - PubMed
-
- Netzer R, Ebneth A, Bischoff U, Pongs O. Screening lead compounds for QT interval prolongation. Drug Discovery Today. 2001;6:78–84. - PubMed
-
- Hancox J, McPate M, Harchi AE, Zhang Y. The hERG potassium channel and hERG screening for drug-induced torsades de pointes. Pharmacology and Therapeutics. 2008;119:118–132. - PubMed
-
- Ficker E, Kuryshev Y, Dennis A, Obejero-Paz C, Lu W, Hawryluk P, Wible B, Brown A. Mechanisms of arsenic-induced prolongation of cardiac repolarization. Molecular Pharmacology. 2004;66:33–44. - PubMed
-
- Traebert M, Dumotier B, Meister L, Hoffmann P, Dominguez-Estevez M, Suter W. Inhibition of hERG K+ currents by antimalarial drugs in stably transfected HEK293 cells. European Journal of Pharmacology. 2004;484:41–48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

