Distinct roles of GCN5/PCAF-mediated H3K9ac and CBP/p300-mediated H3K18/27ac in nuclear receptor transactivation
- PMID: 21131905
- PMCID: PMC3025463
- DOI: 10.1038/emboj.2010.318
Distinct roles of GCN5/PCAF-mediated H3K9ac and CBP/p300-mediated H3K18/27ac in nuclear receptor transactivation
Abstract
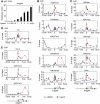
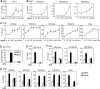
Histone acetyltransferases (HATs) GCN5 and PCAF (GCN5/PCAF) and CBP and p300 (CBP/p300) are transcription co-activators. However, how these two distinct families of HATs regulate gene activation remains unclear. Here, we show deletion of GCN5/PCAF in cells specifically and dramatically reduces acetylation on histone H3K9 (H3K9ac) while deletion of CBP/p300 specifically and dramatically reduces acetylations on H3K18 and H3K27 (H3K18/27ac). A ligand for nuclear receptor (NR) PPARδ induces sequential enrichment of H3K18/27ac, RNA polymerase II (Pol II) and H3K9ac on PPARδ target gene Angptl4 promoter, which correlates with a robust Angptl4 expression. Inhibiting transcription elongation blocks ligand-induced H3K9ac, but not H3K18/27ac, on the Angptl4 promoter. Finally, we show GCN5/PCAF and GCN5/PCAF-mediated H3K9ac correlate with, but are surprisingly dispensable for, NR target gene activation. In contrast, CBP/p300 and their HAT activities are essential for ligand-induced Pol II recruitment on, and activation of, NR target genes. These results highlight the substrate and site specificities of HATs in cells, demonstrate the distinct roles of GCN5/PCAF- and CBP/p300-mediated histone acetylations in gene activation, and suggest an important role of CBP/p300-mediated H3K18/27ac in NR-dependent transcription.
Conflict of interest statement
The authors declare that they have no conflict of interest. The views presented in this article do not necessarily reflect those of the US Food and Drug Administration.
Figures




References
-
- Balasubramanyam K, Varier RA, Altaf M, Swaminathan V, Siddappa NB, Ranga U, Kundu TK (2004) Curcumin, a novel p300/CREB-binding protein-specific inhibitor of acetyltransferase, represses the acetylation of histone/nonhistone proteins and histone acetyltransferase-dependent chromatin transcription. J Biol Chem 279: 51163–51171 - PubMed
-
- Barski A, Cuddapah S, Cui K, Roh T-Y, Schones DE, Wang Z, Wei G, Chepelev I, Zhao K (2007) High-resolution profiling of histone methylations in the human genome. Cell 129: 823–837 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

