Development of asymmetric inhibition underlying direction selectivity in the retina
- PMID: 21131947
- PMCID: PMC3974627
- DOI: 10.1038/nature09600
Development of asymmetric inhibition underlying direction selectivity in the retina
Abstract
Establishing precise synaptic connections is crucial to the development of functional neural circuits. The direction-selective circuit in the retina relies upon highly selective wiring of inhibitory inputs from starburst amacrine cells (SACs) onto four subtypes of ON-OFF direction-selective ganglion cells (DSGCs), each preferring motion in one of four cardinal directions. It has been reported in rabbit that the SACs on the 'null' sides of DSGCs form functional GABA (γ-aminobutyric acid)-mediated synapses, whereas those on the preferred sides do not. However, it is not known how the asymmetric wiring between SACs and DSGCs is established during development. Here we report that in transgenic mice with cell-type-specific labelling, the synaptic connections from SACs to DSGCs were of equal strength during the first postnatal week, regardless of whether the SAC was located on the preferred or null side of the DSGC. However, by the end of the second postnatal week, the strength of the synapses made from SACs on the null side of a DSGC significantly increased whereas those made from SACs located on the preferred side remained constant. Blocking retinal activity by intraocular injections of muscimol or gabazine during this period did not alter the development of direction selectivity. Hence, the asymmetric inhibition between the SACs and DSGCs is achieved by a developmental program that specifically strengthens the GABA-mediated inputs from SACs located on the null side, in a manner not dependent on neural activity.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

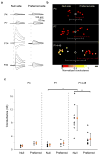
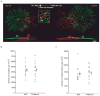

Comment in
-
Developmental neuroscience: asymmetric inhibition.Nat Rev Neurosci. 2011 Feb;12(2):64. doi: 10.1038/nrn2982. Nat Rev Neurosci. 2011. PMID: 21309095 No abstract available.
References
-
- Euler T, Detwiler PB, Denk W. Directionally selective calcium signals in dendrites of starburst amacrine cells. Nature. 2002;418:845–852. - PubMed
-
- Demb JB. Cellular mechanisms for direction selectivity in the retina. Neuron. 2007;55:179–186. - PubMed
-
- Fried SI, Munch TA, Werblin FS. Mechanisms and circuitry underlying directional selectivity in the retina. Nature. 2002;420:411–414. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

