Shotgun glycomics: a microarray strategy for functional glycomics
- PMID: 21131969
- PMCID: PMC3074519
- DOI: 10.1038/nmeth.1540
Shotgun glycomics: a microarray strategy for functional glycomics
Abstract
Major challenges of glycomics are to characterize a glycome and identify functional glycans as ligands for glycan-binding proteins (GBPs). To address these issues we developed a general strategy termed shotgun glycomics. We focus on glycosphingolipids (GSLs), a class of glycoconjugates that is challenging to study, recognized by toxins, antibodies and GBPs. We derivatized GSLs extracted from cells with a heterobifunctional fluorescent tag suitable for covalent immobilization. We separated fluorescent GSLs by multidimensional chromatography, quantified them and coupled them to glass slides to create GSL shotgun microarrays. Then we interrogated the microarrays with cholera toxin, antibodies and sera from individuals with Lyme disease to identify biologically relevant GSLs that we subsequently characterized by mass spectrometry. Shotgun glycomics incorporating GSLs and potentially glycoprotein-derived glycans is an approach for accessing the complex glycomes of animal cells and is a strategy for focusing structural analyses on functionally important glycans.
Figures


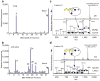

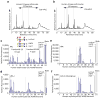
Comment in
-
At last, functional glycomics.Nat Methods. 2011 Jan;8(1):55-7. doi: 10.1038/nmeth0111-55. Nat Methods. 2011. PMID: 21191374 No abstract available.
Similar articles
-
Design of a covalently bonded glycosphingolipid microarray.Glycoconj J. 2012 Jan;29(1):1-12. doi: 10.1007/s10719-011-9359-9. Epub 2011 Nov 22. Glycoconj J. 2012. PMID: 22102144
-
Chemistry of natural glycan microarrays.Curr Opin Chem Biol. 2014 Feb;18:70-7. doi: 10.1016/j.cbpa.2014.01.001. Epub 2014 Jan 30. Curr Opin Chem Biol. 2014. PMID: 24487062 Free PMC article. Review.
-
History and future of shotgun glycomics.Biochem Soc Trans. 2019 Feb 28;47(1):1-11. doi: 10.1042/BST20170487. Epub 2019 Jan 9. Biochem Soc Trans. 2019. PMID: 30626702 Review.
-
A platform for the structural characterization of glycans enzymatically released from glycosphingolipids extracted from tissue and cells.Rapid Commun Mass Spectrom. 2015 Apr 15;29(7):545-61. doi: 10.1002/rcm.7130. Rapid Commun Mass Spectrom. 2015. PMID: 26212272
-
Qualitative and quantitative cellular glycomics of glycosphingolipids based on rhodococcal endoglycosylceramidase-assisted glycan cleavage, glycoblotting-assisted sample preparation, and matrix-assisted laser desorption ionization tandem time-of-flight mass spectrometry analysis.J Biol Chem. 2011 Dec 2;286(48):41669-41679. doi: 10.1074/jbc.M111.301796. Epub 2011 Sep 30. J Biol Chem. 2011. PMID: 21965662 Free PMC article.
Cited by
-
"Stuck on sugars - how carbohydrates regulate cell adhesion, recognition, and signaling".Glycoconj J. 2019 Aug;36(4):241-257. doi: 10.1007/s10719-019-09876-0. Epub 2019 Jul 2. Glycoconj J. 2019. PMID: 31267247 Free PMC article.
-
Lipidomics of glycosphingolipids.Metabolites. 2012 Feb 2;2(1):134-64. doi: 10.3390/metabo2010134. Metabolites. 2012. PMID: 24957371 Free PMC article.
-
SARS-CoV-2 and other coronaviruses bind to phosphorylated glycans from the human lung.Virology. 2021 Oct;562:142-148. doi: 10.1016/j.virol.2021.07.012. Epub 2021 Jul 23. Virology. 2021. PMID: 34325286 Free PMC article.
-
Factors Affecting Anti-Glycan IgG and IgM Repertoires in Human Serum.Sci Rep. 2016 Jan 19;6:19509. doi: 10.1038/srep19509. Sci Rep. 2016. PMID: 26781493 Free PMC article.
-
Carbohydrate microarrays.Chem Soc Rev. 2013 May 21;42(10):4310-26. doi: 10.1039/c2cs35401b. Epub 2012 Nov 28. Chem Soc Rev. 2013. PMID: 23192235 Free PMC article. Review.
References
-
- Varki A, et al. Essentials of Glycobiology. 2. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, N.Y: 2009. - PubMed
-
- Lowe JB, Marth JD. A genetic approach to Mammalian glycan function. Annu Rev Biochem. 2003;72:643–691. - PubMed
-
- Freeze HH, Aebi M. Altered glycan structures: the molecular basis of congenital disorders of glycosylation. Curr Opin Struct Biol. 2005;15:490–498. - PubMed
-
- Cummings RD. The repertoire of glycan determinants in the human glycome. Mol Biosyst. 2009;5:1087–1104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

