β₂-microglobulin forms three-dimensional domain-swapped amyloid fibrils with disulfide linkages
- PMID: 21131979
- PMCID: PMC3058263
- DOI: 10.1038/nsmb.1948
β₂-microglobulin forms three-dimensional domain-swapped amyloid fibrils with disulfide linkages
Abstract
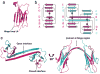
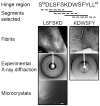
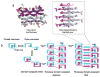
β₂-microglobulin (β₂m) is the light chain of the type I major histocompatibility complex. It deposits as amyloid fibrils within joints during long-term hemodialysis treatment. Despite the devastating effects of dialysis-related amyloidosis, full understanding of how fibrils form from soluble β₂m remains elusive. Here we show that β₂m can oligomerize and fibrillize via three-dimensional domain swapping. Isolating a covalently bound, domain-swapped dimer from β₂m oligomers on the pathway to fibrils, we were able to determine its crystal structure. The hinge loop that connects the swapped domain to the core domain includes the fibrillizing segment LSFSKD, whose atomic structure we also determined. The LSFSKD structure reveals a class 5 steric zipper, akin to other amyloid spines. The structures of the dimer and the zipper spine fit well into an atomic model for this fibrillar form of β₂m, which assembles slowly under physiological conditions.
Figures
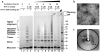
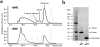
References
-
- Westermark P, et al. Amyloid: toward terminology clarification. Report from the Nomenclature Committee of the International Society of Amyloidosis. Amyloid. 2005;12:1–4. - PubMed
-
- Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem. 2006;75:333–66. - PubMed
-
- Makin OS, Serpell LC. Structures for amyloid fibrils. Febs J. 2005;272:5950–61. - PubMed
-
- Sawaya MR, et al. Atomic structures of amyloid cross-β spines reveal varied steric zippers. Nature. 2007;447:453–7. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

