Radiation-induced intercellular signaling mediated by cytochrome-c via a p53-dependent pathway in hepatoma cells
- PMID: 21132005
- PMCID: PMC3070628
- DOI: 10.1038/onc.2010.567
Radiation-induced intercellular signaling mediated by cytochrome-c via a p53-dependent pathway in hepatoma cells
Abstract
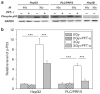
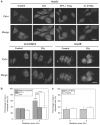
The tumor suppressor p53 has a crucial role in cellular response to DNA damage caused by ionizing radiation, but it is still unclear whether p53 can modulate radiation-induced bystander effects (RIBE). In the present work, three different hepatoma cell lines, namely HepG2 (wild p53), PLC/PRF/5 (mutation p53) and Hep3B (p53 null), were irradiated with γ-rays and then co-cultured with normal Chang liver cell (wild p53) in order to elucidate the mechanisms of RIBE. Results showed that the radiosensitivity of HepG2 cells was higher than that of PLC/PRF/5 and Hep3B cells. Only irradiated HepG2 cells, rather than irradiated PLC/PRF/5 or Hep3B cells, could induce bystander effect of micronuclei (MN) formation in the neighboring Chang liver cells. When HepG2 cells were treated with 20 μM pifithrin-α, an inhibitor of p53 function, or 5 μM cyclosporin A (CsA), an inhibitor of cytochrome-c release from mitochondria, the MN induction in bystander Chang liver cells was diminished. In fact, it was found that after irradiation, cytochrome-c was released from mitochondria into the cytoplasm only in HepG2 cells in a p53-dependent manner, but not in PLC/PRF/5 and Hep3B cells. Interestingly, when 50 μg/ml exogenous cytochrome-c was added into cell co-culture medium, RIBE was significantly triggered by irradiated PLC/PRF/5 and Hep3B cells, which previously failed to provoke a bystander effect. In addition, this exogenous cytochrome-c also partly recovered the RIBE induced by irradiated HepG2 cells even with CsA treatment. Our results provide new evidence that the RIBE can be modulated by the p53 status of irradiated hepatoma cells and that a p53-dependent release of cytochrome-c may be involved in the RIBE.
Figures




Similar articles
-
Alpha particle-induced bystander effect is mediated by ROS via a p53-dependent SCO2 pathway in hepatoma cells.Int J Radiat Biol. 2013 Dec;89(12):1028-34. doi: 10.3109/09553002.2013.817706. Epub 2013 Jul 24. Int J Radiat Biol. 2013. PMID: 23786650
-
Cytochrome-c mediated a bystander response dependent on inducible nitric oxide synthase in irradiated hepatoma cells.Br J Cancer. 2012 Feb 28;106(5):889-95. doi: 10.1038/bjc.2012.9. Epub 2012 Jan 24. Br J Cancer. 2012. PMID: 22274409 Free PMC article.
-
Radiation enhances the invasiveness of irradiated and nonirradiated bystander hepatoma cells through a VEGF-MMP2 pathway initiated by p53.Radiat Res. 2013 Oct;180(4):389-97. doi: 10.1667/RR3355.1. Epub 2013 Sep 23. Radiat Res. 2013. PMID: 24059678
-
SirT1 knockdown potentiates radiation-induced bystander effect through promoting c-Myc activity and thus facilitating ROS accumulation.Mutat Res. 2015 Feb;772:23-9. doi: 10.1016/j.mrfmmm.2014.12.010. Epub 2015 Jan 3. Mutat Res. 2015. PMID: 25772107
-
[Radiation-induced bystander effect: the important part of ionizing radiation response. Potential clinical implications].Postepy Hig Med Dosw (Online). 2009 Aug 18;63:377-88. Postepy Hig Med Dosw (Online). 2009. PMID: 19724078 Review. Polish.
Cited by
-
Coupling of cell fate selection model enhances DNA damage response and may underlie BE phenomenon.IET Syst Biol. 2020 Apr;14(2):96-106. doi: 10.1049/iet-syb.2019.0081. IET Syst Biol. 2020. PMID: 32196468 Free PMC article.
-
Exosomes in Cancer Radioresistance.Front Oncol. 2019 Sep 6;9:869. doi: 10.3389/fonc.2019.00869. eCollection 2019. Front Oncol. 2019. PMID: 31555599 Free PMC article. Review.
-
The cross-talk between Bax, Bcl2, caspases, and DNA damage in bystander HepG2 cells is regulated by γ-radiation dose and time of conditioned media transfer.Apoptosis. 2022 Apr;27(3-4):184-205. doi: 10.1007/s10495-022-01713-4. Epub 2022 Jan 25. Apoptosis. 2022. PMID: 35076828
-
Influence of P53 on the radiotherapy response of hepatocellular carcinoma.Clin Mol Hepatol. 2015 Sep;21(3):257-67. doi: 10.3350/cmh.2015.21.3.257. Epub 2015 Sep 30. Clin Mol Hepatol. 2015. PMID: 26527121 Free PMC article.
-
Biological mechanisms of gold nanoparticle radiosensitization.Cancer Nanotechnol. 2017;8(1):2. doi: 10.1186/s12645-017-0026-0. Epub 2017 Feb 2. Cancer Nanotechnol. 2017. PMID: 28217176 Free PMC article. Review.
References
-
- Ain JF, Gouillat C, Bertrand S, Fourel I, Guillaud M, Saguier G, et al. Human hepatocellular carcinoma transplanted in nude mice: a relevant experimental model to assess tumoral destruction by alcoholization. J Surg Res. 1994;57:366–372. - PubMed
-
- Atlante A, Calissano P, Bobba A, Azzariti A, Marra E, Passarella S. Cytochrome c is released from mitochondria in a reactive oxygen species (ROS)-dependent fashion and can operate as a ROS scavenger and as a respiratory substrate in cerebellar neurons undergoing excitotoxic death. J Biol Chem. 2000;275:37159–37166. - PubMed
-
- Azzam EI, de Toledo SM, Little JB. Expression of CONNEXIN43 is highly sensitive to ionizing radiation and other environmental stresses. Cancer Res. 2003a;63:7128–7135. - PubMed
-
- Azzam EI, de Toledo SM, Little B. Oxidative metabolism, gap junctions and the ionizing radiation-induced bystander effect. Oncogene. 2003b;22:7050–7057. - PubMed
-
- Bartek J, Bartkova J, Vojtesek B, Staskova Z, Lukas J, Rejthar A, et al. Aberrant expression of the p53 oncoprotein is a common feature of a wide spectrum of human malignancies. Oncogene. 1991;6:1699–1703. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

