Chromatin modification acts as a memory for systemic acquired resistance in the plant stress response
- PMID: 21132017
- PMCID: PMC3024125
- DOI: 10.1038/embor.2010.186
Chromatin modification acts as a memory for systemic acquired resistance in the plant stress response
Abstract
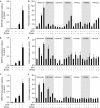
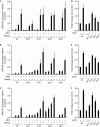
Priming of defence genes for amplified response to secondary stress can be induced by application of the plant hormone salicylic acid or its synthetic analogue acibenzolar S-methyl. In this study, we show that treatment with acibenzolar S-methyl or pathogen infection of distal leaves induce chromatin modifications on defence gene promoters that are normally found on active genes, although the genes remain inactive. This is associated with an amplified gene response on challenge exposure to stress. Mutant analyses reveal a tight correlation between histone modification patterns and gene priming. The data suggest a histone memory for information storage in the plant stress response.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
-
- Asai T, Tena G, Plotnikova J, Willmann MR, Chiu WL, Gomez-Gomez L, Boller T, Ausubel FM, Sheen J (2002) MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 415: 977–983 - PubMed
-
- Bernstein BE et al. (2006) A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell 125: 315–326 - PubMed
-
- Chen TY, Lei MG, Suzuki T, Morrison DC (1992) Lipopolysaccharide receptors and signal transduction pathways in mononuclear phagocytes. Curr Top Micro Immunol 181: 169–188 - PubMed
-
- Chomczynski P (1993) A reagent for the single-step simultaneous isolation of RNA, DNA and proteins from cell and tissue samples. Biotechniques 15: 532–534 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

