Emerging concepts and approaches for chemokine-receptor drug discovery
- PMID: 21132095
- PMCID: PMC2995586
- DOI: 10.1517/17460441.2010.525633
Emerging concepts and approaches for chemokine-receptor drug discovery
Abstract
Importance of the field: Chemokine receptors are most noted for their role in cell migration. However, inappropriate utilization or regulation of these receptors is implicated in many inflammatory diseases, cancer and HIV, making them important drug targets.
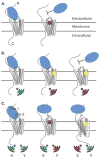
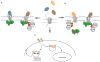
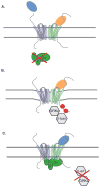
Areas covered in this review: Allostery, oligomerization and ligand bias are presented as they pertain to chemokine receptors and their associated pathologies.Specific examples of each are described from the recent literature and their implications are discussed in terms of drug discovery efforts targeting chemokine receptors.
What the reader will gain: Insight into the expanding view of the multitude of pharmacological variables that need to be considered or that may be exploited in chemokine receptor drug discovery.
Take home message: Since 2007, two drugs targeting chemokine receptors have been approved by the FDA, Maraviroc for preventing HIV infection and Mozobil™ for hematopoietic stem cell mobilization. While these successes permit optimism for chemokine receptors as drug targets, only recently has the complexity of this system begun to be appreciated. The concepts of allosteric inhibitors, biased ligands and functional selectivity raise the possibility that drugs with precisely-defined properties can be developed. Other complexities such as receptor oligomerization and tissue-specific functional states of receptors also offer opportunities for increased target and response specificity, although it will be more challenging to translate these ideas into approved therapeutics compared to traditional approaches.
Keywords: G protein-coupled receptors; allosteric inhibitors; chemokine receptors; drug discovery; functional selectivity; ligand bias; oligomerization.
Conflict of interest statement
M O’Hayre was supported by a California Breast Cancer Research Program Dissertation Award (14GB-0147) while CL Salanga was supported by a Ruth L. Kirschstein NIGMS MARC Predoctoral Fellowship (F31). DJ Hamel was supported by an NIH Postdoctoral Fellowship (F32 GM083463), while T Handel received awards from NIAID (RO1-AI37113), NIGMS (RO1-GM081763) and was supported by the Lymphoma Foundation
Figures
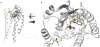
Similar articles
-
Anti-chemokine small molecule drugs: a promising future?Expert Opin Investig Drugs. 2010 Mar;19(3):345-55. doi: 10.1517/13543780903535867. Expert Opin Investig Drugs. 2010. PMID: 20113217 Review.
-
Pharmacological modulation of chemokine receptor function.Br J Pharmacol. 2012 Mar;165(6):1617-1643. doi: 10.1111/j.1476-5381.2011.01551.x. Br J Pharmacol. 2012. PMID: 21699506 Free PMC article. Review.
-
Allosteric inhibition of g-protein coupled receptor oligomerization: strategies and challenges for drug development.Curr Top Med Chem. 2014;14(15):1842-63. doi: 10.2174/1568026614666140901130843. Curr Top Med Chem. 2014. PMID: 25175995 Review.
-
What Do Structures Tell Us About Chemokine Receptor Function and Antagonism?Annu Rev Biophys. 2017 May 22;46:175-198. doi: 10.1146/annurev-biophys-051013-022942. Annu Rev Biophys. 2017. PMID: 28532213 Free PMC article. Review.
-
Dimerization of chemokine receptors in living cells: key to receptor function and novel targets for therapy.Drug Discov Today. 2008 Jul;13(13-14):625-32. doi: 10.1016/j.drudis.2008.04.004. Epub 2008 Jun 3. Drug Discov Today. 2008. PMID: 18598920 Review.
Cited by
-
The role of chemokines in Guillain-Barré syndrome.Muscle Nerve. 2013 Sep;48(3):320-30. doi: 10.1002/mus.23829. Epub 2013 Jul 27. Muscle Nerve. 2013. PMID: 23447114 Free PMC article. Review.
-
Circulating chemokine ligand levels before and after successful kidney transplantation.J Inflamm (Lond). 2016 Oct 26;13:32. doi: 10.1186/s12950-016-0141-4. eCollection 2016. J Inflamm (Lond). 2016. PMID: 27795695 Free PMC article.
-
Co-receptor signaling in the pathogenesis of neuroHIV.Retrovirology. 2021 Aug 24;18(1):24. doi: 10.1186/s12977-021-00569-x. Retrovirology. 2021. PMID: 34429135 Free PMC article. Review.
-
Grapefruit-Derived Nanovectors Use an Activated Leukocyte Trafficking Pathway to Deliver Therapeutic Agents to Inflammatory Tumor Sites.Cancer Res. 2015 Jun 15;75(12):2520-9. doi: 10.1158/0008-5472.CAN-14-3095. Epub 2015 Apr 16. Cancer Res. 2015. PMID: 25883092 Free PMC article.
-
Nematode asparaginyl-tRNA synthetase resolves intestinal inflammation in mice with T-cell transfer colitis.Clin Vaccine Immunol. 2013 Feb;20(2):276-81. doi: 10.1128/CVI.00594-12. Epub 2012 Dec 19. Clin Vaccine Immunol. 2013. PMID: 23254300 Free PMC article.
References
-
- Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392(6676):565–568. - PubMed
-
- Murphy PM. The molecular biology of leukocyte chemoattractant receptors. Annu Rev Immunol. 1994;12:593–633. - PubMed
-
- Baggiolini M. Chemokines in pathology and medicine. J Intern Med. 2001;250(2):91–104. - PubMed
-
- Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000;18:217–242. - PubMed
-
- Wells TN, Proudfoot AE, Power CA. Chemokine receptors and their role in leukocyte activation. Immunol Lett. 1999;65(1–2):35–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
