Invasion and destruction of a murine fibrosarcoma by Salmonella-induced effector CD8 T cells as a therapeutic intervention against cancer
- PMID: 21132428
- PMCID: PMC11028716
- DOI: 10.1007/s00262-010-0950-x
Invasion and destruction of a murine fibrosarcoma by Salmonella-induced effector CD8 T cells as a therapeutic intervention against cancer
Abstract
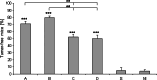
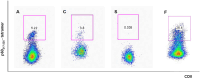
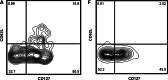
We have developed a new vaccination strategy by using the Salmonella type III secretion system (T3SS) to translocate heterologous antigens into the cytosol of host cells. This leads to an efficient antigen-specific CD8 T cell induction. Recently, we have demonstrated the use of Salmonella's T3SS for the immunoprophylaxis of a solid tumor. The murine fibrosarcoma WEHI 164 was transfected with the DNA sequence encoding the MHC class I-peptide p60(217-225) from Listeria monocytogenes. In the present study, we used this tumor model to investigate the potential of vaccination with recombinant Salmonella in a therapeutic setting. BALB/c mice were subcutaneously challenged with WEHI-p60 cells. Simultaneously or 4 days later, these mice received either an orogastric or intravenous immunization with Salmonella translocating p60. Interestingly, 71-80% of the intravenously and 50-52% of the orogastrically immunized mice showed a complete tumor regression after 14 days. In addition, the distribution of tetramer-positive p60(217-225)-specific CD8 T cell subpopulations in blood and tumor tissue was analyzed. Co-staining with CD62L and CD127 revealed that the frequencies of p60(217-225)-specific effector and effector memory CD8 T cells in blood and in fibrosarcoma tissue were related to the kinetics of tumor regression. In summary, our study demonstrates that therapeutic vaccination with Salmonella leads to efficient induction of tumor-invading effector CD8 T cells that may result in significant tumor regression.
Figures



References
-
- Berchtold C, Panthel K, Jellbauer S, Köhn B, Roider E, Partilla M, Heesemann J, Endres S, Bourquin C, Rüssmann H. Superior protective immunity against murine listeriosis by combined vaccination with CpG DNA and recombinant Salmonella . Infect Immun. 2009;77:5501–5508. doi: 10.1128/IAI.00700-09. - DOI - PMC - PubMed
-
- Coley WB. The treatment of malignant tumors by repeated inoculations of erysipelas. With a report of ten original cases. Clin Orthop Relat Res. 1893;262:3–11. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

