Combination of stromal cell-derived factor-1 and collagen-glycosaminoglycan scaffold delays contraction and accelerates reepithelialization of dermal wounds in wild-type mice
- PMID: 21134036
- PMCID: PMC3167377
- DOI: 10.1111/j.1524-475X.2010.00646.x
Combination of stromal cell-derived factor-1 and collagen-glycosaminoglycan scaffold delays contraction and accelerates reepithelialization of dermal wounds in wild-type mice
Abstract
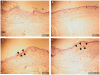
While dermal substitutes can mitigate scarring and wound contraction, a significant drawback of current dermal replacement technologies is the apparent delay in vascular ingrowth compared with conventional skin grafts. Herein, we examined the effect of the chemokine stromal cell-derived factor-1 (SDF-1) on the performance of a porous collagen-glycosaminoglycan dermal analog in excisional wounds in mice. C57BL/6 mice with 1 cm × 1 cm dorsal full-thickness wounds were covered with a collagen-glycosaminoglycan scaffold, followed by four daily topical applications of 1 μg SDF-1 or phosphate-buffered saline vehicle. Some animals were also pretreated with five daily doses of 300 mg/kg granulocyte colony-stimulating factor. Animals treated with SDF-1 and no granulocyte colony-stimulating factor reepithelialized 36% faster than vehicle controls (16 vs. 25 days), and exhibited less wound contraction on postwounding day 18 (∼ 35% greater wound area) plus three-fold longer neoepidermis formed than controls. Conversely, granulocyte colony-stimulating factor promoted contraction and no epidermal regeneration. Early (postwounding Day 3) inflammatory cell infiltration in the SDF-1-treated group was 86% less, while the fraction of proliferating cells (positive Ki67 staining) was 32% more, when compared with controls. These results suggest that SDF-1 simultaneously delays contraction and promotes reepithelialization and may improve the wound-healing performance of skin substitutes.
© 2010 by the Wound Healing Society.
Figures



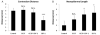

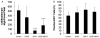

References
-
- Rhett JM, Ghatnekar GS, Palatinus JA, O’Quinn M, Yost MJ, Gourdie RG. Novel therapies for scar reduction and regenerative healing of skin wounds. Trends Biotechnol. 2008;26:173–80. - PubMed
-
- Auger FA, Berthod F, Moulin V, Pouliot R, Germain L. Tissue-engineered skin substitutes: from in vitro constructs to in vivo applications. Biotechnol Appl Biochem. 2004;39(Part 3):263–75. - PubMed
-
- Bargues L, Boyer S, Leclerc T, Duhamel P, Bey E. Incidence and microbiology of infectious complications with the use of artificial skin Integra in burns. Ann Chir Plast Esthet. 2009;54:533–9. - PubMed
-
- Orgill DP. Excision and skin grafting of thermal burns. N Engl J Med. 2009;360:893–901. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

